Sansure Biotech Inc. has made a breakthrough in the field of chemiluminescence immunoassay. Recently, six products independently developed by Accucise Diagnostics Inc., a company controlled by Sansure, obtained medical device registration certificates. These medical device registration certificates were issued by Jiangsu Medical Products Administration (JSMPA). The approved products include the reagents and quality controls of Procalcitonin Detection Kit (Electrochemiluminescence Method) […]
On May 9th, Sansure Biotech Inc. issued an investment announcement that jointly establishing Hunan Sansure-Accucise Biotech Co., Ltd. with the Industrial Funds in order to further improve the company’s comprehensive strategic layout in the field of immune diagnosis, especially chemiluminescence. After the establishment of the joint venture company, it will become the investment corpus of Shenzhen Accucise Diagnostics Inc. through […]
Jiangsu Yang Shengyuan Biotech Co. Ltd., a joint venture, was jointly established by Sansure Biotech and Singlera recently, in which Sansure Biotech holds 40% and Singlera holds 60%. Based on the advantages of its two shareholders, the joint venture will work on livelihood projects in accordance with national policies. In order to contribute to the improvement of the national health […]
Blood-borne infections are caused by pathogens that are carried in the blood, specifically HBV, HCV and HIV. Germs that can have a long-lasting presence in human blood and other body fluids are called bloodborne pathogens, resulting in organ damages.
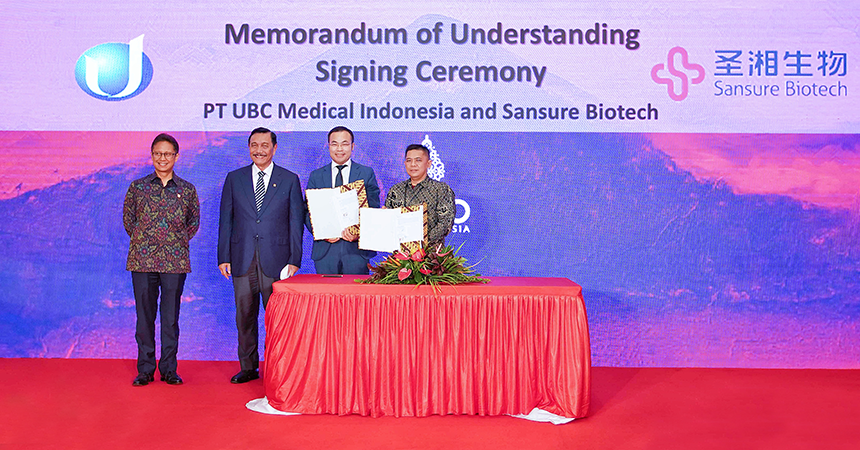
On the afternoon of November 13, witnessed by Budi Gunadi Sadikin, Indonesia’s Minister of Health, and Luhut Binsar Pandjaitan, Indonesia’s Coordinating Minister of Maritime Affairs and Investment, Sansure Biotech signed a memorandum on localized manufacturing with its Indonesian partner UBC, introducing Sansure’s molecular diagnostic techniques to Indonesia and starting localized manufacturing. This is a milestone of Sansure’s international development and […]