Jiangsu Yang Shengyuan Biotech Co. Ltd., a joint venture, was jointly established by Sansure Biotech and Singlera recently, in which Sansure Biotech holds 40% and Singlera holds 60%. Based on the advantages of its two shareholders, the joint venture will work on livelihood projects in accordance with national policies. In order to contribute to the improvement of the national health […]
Blood-borne infections are caused by pathogens that are carried in the blood, specifically HBV, HCV and HIV. Germs that can have a long-lasting presence in human blood and other body fluids are called bloodborne pathogens, resulting in organ damages.
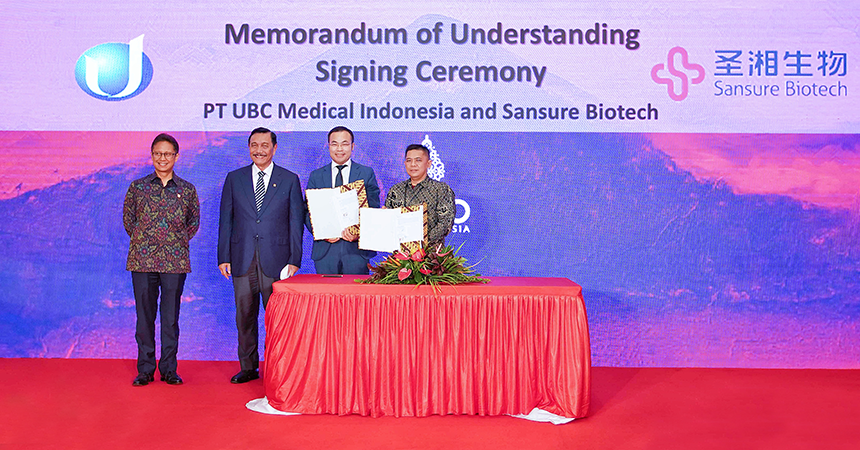
On the afternoon of November 13, witnessed by Budi Gunadi Sadikin, Indonesia’s Minister of Health, and Luhut Binsar Pandjaitan, Indonesia’s Coordinating Minister of Maritime Affairs and Investment, Sansure Biotech signed a memorandum on localized manufacturing with its Indonesian partner UBC, introducing Sansure’s molecular diagnostic techniques to Indonesia and starting localized manufacturing. This is a milestone of Sansure’s international development and […]
Recently, under the guidance of WHO, The Robert Koch Institute (RKI), a German Reference Laboratory for Poxviruses, conducted overall evaluation on eleven PCR kits for the detection of Monkeypox Virus (MPXV) DNA, among which Sansure shows excellent performance in terms of both detection rate and specificity. Since May 2022, the rapid communication of Monkeypox around the world, especially in Europe […]
Recently, the TSure Nucleic Acid Analysis Device independently developed by Sansure obtained CE mark, which will further meet and expand the global testing market demands at all levels, and serve the construction of public health prevention and control system in every country better. Sansure’s TSure Nucleic Acid Analysis Device is a portable device that can carry LAMP thermostatic amplification reagent […]