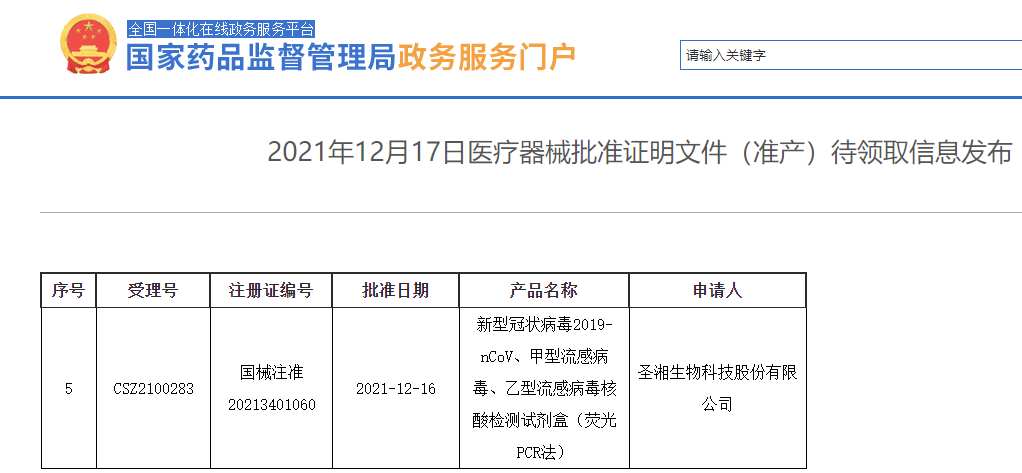
Sansure Biotech’s SARS-CoV-2 and Influenza A/B Virus Nucleic Acid Diagnostic Kit was approved by China NMPA for marketing
The kit developed by Sansure Biotech for nucleic acid detection of SARS-CoV-2, and influenza A & B virus (PCR-Fluorescence Probing) was approved by the National Medical Products Administration (NMPA) for marketing on December 17. It has also received CE mark and is expected to provide critical support for global COVID-19 prevention and control.
According to WHO estimates, influenza causes 3 to 5 million severe cases and 290,000 to 650,000 respiratory disease-related deaths worldwide each year. China’s influenza surveillance data show that the southern provinces have seen a significant increase in influenza activity since September this year. According to experts from the China CDC there may be a risk of an influenza epidemic this winter and next spring, and sporadic outbreaks of COVID-19 in different areas of the country may bring the combined risk of SARS-CoV-2 and influenza, making the prevention and fight against epidemics face a more serious and complex situation.
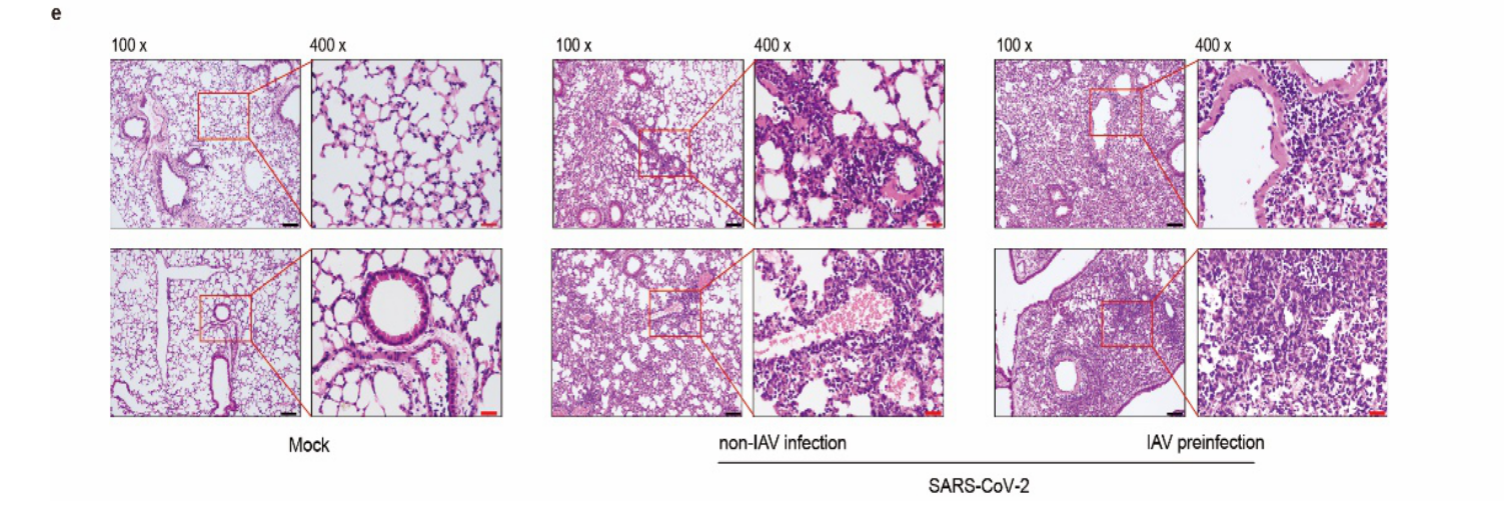

Studies have shown that co-infection with influenza and SARS-CoV-2 may cause more severe pulmonary pathological damage and higher viral load of SARS-CoV-2. It was found in a retrospective analysis of hospital data that co-infection with SARS-CoV-2 in patients infected with influenza B virus further increased the viral load, leading to an increased chance of severe disease progression, and severe pulmonary pathological damage prolonged the disease, while higher viral load of SARS-CoV-2 would make individual transmission more likely and epidemic control more difficult.
In particular, it is difficult to distinguish influenza from COVID-19 patients as they have similar clinical symptoms, and it is often confused with respiratory infections caused by other pathogens in clinical practice. Therefore, it is explicitly mentioned in the Protocol for Diagnosis and Treatment of Influenza and the COVID-19 Prevention and Control Plan that importance should be attached to the etiological identification of the two viruses to guide accurate diagnosis and treatment.
In response to normalized epidemic prevention and control as well as pain points and difficulties in identification of SARS-CoV-2 and influenza in the autumn and winter influenza season, Sansure Biotech has finally developed a multiplex nucleic acid detection kit for “SARS-CoV-2, influenza A and influenza B” based on multiplex fluorescent PCR technology after extensive validation through its intensive efforts in research on SARS-CoV-2, influenza and their characteristics.
The detection kit can simultaneously identify SARS-CoV-2, influenza A virus and influenza B virus in the same tube of reaction solution in one test, matching the sample dispensing robot and automated sample extraction system to cover medical institutions at all levels, especially to provide accurate, rapid and accessible full-scene solutions for fever outpatient clinics and epidemic outbreak sites for prevention and control.
Taking the respiratory field as the focus of its business layout 7 years ago, Sansure Biotech has developed more than 30 types of respiratory testing products such as SARS-CoV-2 nucleic acid detection kits, SARS-CoV-2+influenza A&B nucleic acid detection kits, and six respiratory pathogens nucleic acid detection kits, forming a full-scenario overall solution to help prevent and control respiratory infectious diseases. Sansure Biotech will give full play to its advantages in technology and products in the future to improve the “accuracy” and “accessibility” of respiratory pathogen detection, to promote the construction of a prevention-based health strategy and a precision diagnosis and treatment system for early diagnosis and treatment to contribute to public health.





