The Detection Kit for 5 Mutations of SARS-CoV-2 of Sansure Biotech obtained the CE certification
Recently, the Detection Kit for 5 Mutations of SARS-CoV-2 (PCR-Fluorescence Probing) developed by Sansure Biotech have obtained the European Union CE certification. This is another COVID-19 mutations diagnostic product which obtained the EU CE certification after the mutations diagnostic kit for the identification of B.1.1.7 major mutation sites N501Y and HV69-70 del obtained the CE certification.

This product can simultaneously identify the typical mutation sites carried by the British mutant strain B.1.1.7, the South African mutant strain B.1.351, and the Brazilian mutant strain P.1.
At present, the World Health Organization lists the British variant (B.1.1.7), the South African variant (B.1.351/501Y.V2), and the Brazilian variant (P.1/501Y.V3) as viral variant of high concern, and report the monitoring situation of mutant strains every week. As of May 21, a total of 137 countries have reported the discovery of the British variant; 94 countries have reported the discovery of the South African variant, and 56 countries have reported the discovery of the Brazilian variant. These mutant strains carry N501Y, E484K, P681H or K417N/T and other key site mutations, which increase the ability of the virus S protein to bind to the receptor ACE2 and have a certain probability of evading the recognition of neutralizing antibodies, resulting in a greatly improved transmission of the COVID-19 virus. Some studies have shown that it may increase the lethality rate of the COVID-19 virus and the probability of secondary infection.
|
Variant name |
Alias | First discovery |
Typical mutation |
|
| Time |
Location |
|||
|
B. 1. 1. 7 |
20I/501Y. V1 | Sep. 20, 2020 | United Kingdom |
N501Y, P681H and HV69-70del |
|
B. 1. 351 |
20H/501Y. V2 | Sep. 1, 2020 | South Africa |
N501Y, K417N and E484K |
| P. 1 | 20J/501Y. V3 | Nov. 3, 2020 | Brazil |
E484K, N501Y and K417T |
Information on the world’s major COVID-19 viral variant
(Data source: PANGO lineages)
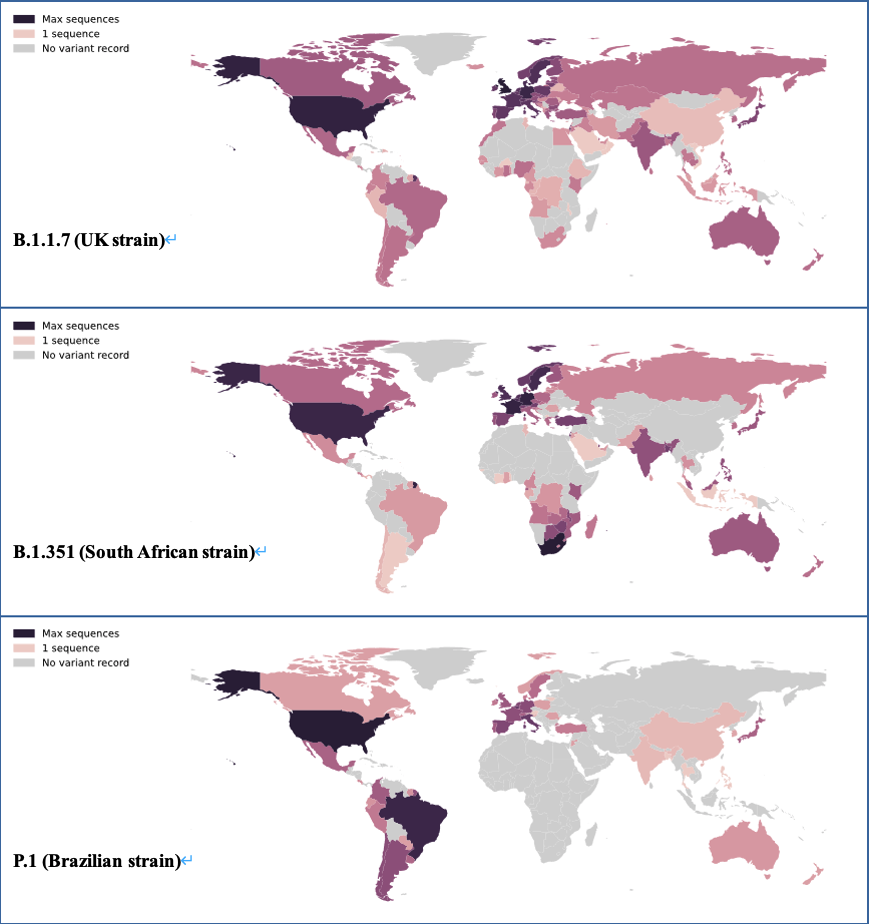
The number of record of mutation sequences of mutant strains in the world
(Data source: PANGO lineages)
The CE-certified product Detection Kit for 5 Mutations of SARS-CoV-2 (PCR-Fluorescence Probing) of Sansure Biotech can simultaneously complete the detection of 5 mutations (N501Y, HV69-70del, K417N, P681H, and E484K) of COVID-19 virus with one test through the multiplex PCR-Fluorescence Probing, realizing the rapid differential diagnosis of mutant virus strains (B.1.1.7, B.1.351, P.1). At the same time, it uses the COVID-19 conservative sequence N gene as the monitoring internal standard, which greatly improves the accuracy of sample test results. This kit will help the rapid identification and monitoring of mutant strains of the COVID-19 virus.

In order to cope with the impact of the Indian COVID-19 virus variant strain on the global epidemic prevention and control, Sansure Biotech has successfully developed a multiplex nucleic acid diagnostic kit to identify the typical mutation sites (L452R, E484Q, P681R) it carries targeted the B.1.617+, which has been designated by the WHO as a variant strain of global concern.
At present, Sansure Biotech has formed a system solution from the rapid detection of the COVID-19 virus to the rapid identification of mutation sites in all scenarios, contributing China’s strength to the prevention and control of the global epidemic.





