Severe Hepatitis of Unknown Origin in Children: Adenovirus or COVID-19 Infection?
Recent studies of severe acute hepatitis of unknown origin in previously healthy youngsters in many countries have resulted in health alerts and a race to uncover the underlying cause. Between April 5 and May 26, 2022, 650 probable cases of acute hepatitis of unknown etiology in children were reported to WHO from 33 countries in 5 WHO regions.
The underlying cause of this severe acute hepatitis remains unknown and under investigation; the cases are more clinically severe, and a greater proportion of them develop acute liver failure, as compared to earlier reports of acute hepatitis in children with unknown origin. However, whereas the condition is still rare, physicians and public health officials are concerned since the current outbreak of hepatitis in children does not appear to be caused by any of the five recognized human hepatitis viruses.
According to a WHO report released on April 23, these viruses had not been detected in any of the reported cases. However, adenovirus, a different type of virus, was found in at least 74 cases of rare childhood hepatitis.
Cause of Hepatitis of Unknown Origin
One leading hypothesis is that adenovirus (a common virus that causes mild cold or flu symptoms) is involved. Adenovirus has been the most commonly detected infectious agent in these mysterious cases in children (found in nearly half of children in the US and two-thirds of cases in the UK). This theory is reinforced by the fact that nearly 25% of the cases shared the same viral serotype, adenovirus F41, and that many of the infants may have never been exposed to adenovirus before the COVID-19 pandemic when infection rates were at an all-time low.
While the adenovirus hypothesis is under investigation, other infectious and/or non-infectious etiologies, along with novel variants of adenovirus and SARS-CoV-2 (particularly the Omicron variant), continue to be explored. Active or recent SARS-CoV-2 infection has been detected in only 15% of children affected in the UK, but serologic studies to establish the incidence of prior infection will provide crucial information about the likelihood of a post-infectious SARS-CoV-2 syndrome.
In Europe, of 19 cases with SARS-CoV-2 serology results were positive. However, serological evidence will be hard to interpret due to the high infection rate, especially during the Omicron wave. There is no connection to the COVID-19 vaccine because nearly all of the children who were affected were too young to be vaccinated.
Adenovirus has been found in at least 74 cases, with 18 of those characterized as F type 41 based on information from molecular testing [1]. SARS-CoV-2 was found in 20 of the people who were examined. In addition, 19 cases of SARS-CoV-2 and adenovirus co-infection were reported.
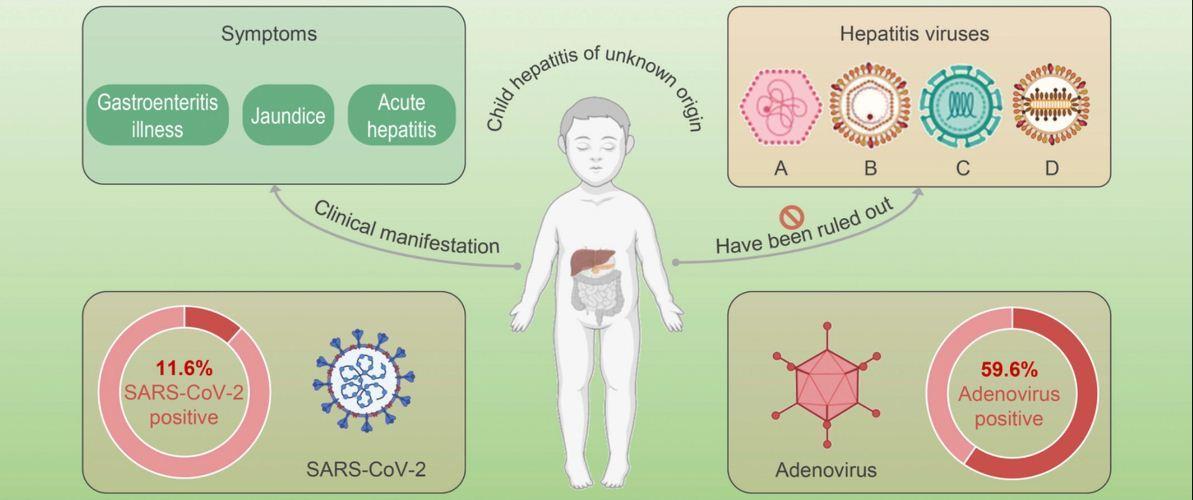
Figure 2: Clinical manifestation of child hepatitis of unknown origin
In Response to Unknown Origin Children Hepatitis, Coronavirus Test, and Adenovirus Test Should Be Emphasized
Centers for Disease Control and Prevention (CDC) investigates a cluster of children with hepatitis and adenovirus infection. CDC suggested that clinicians in the United States who encounter pediatric patients with hepatitis of unknown etiology consider adenovirus testing and report cases of hepatitis of unknown etiology to state public health authorities and the CDC. While adenovirus type 41 infection has been linked to hepatitis in immunocompromised children in the past, it is not known to cause hepatitis in healthy children.
Coronavirus and Adenovirus Nucleic Acid Test in Detecting Unknown Origin Hepatitis
- Clinicians should consider coronavirus and adenovirus testing in pediatric patients with hepatitis of unknown etiology. It is preferable to use a Nucleic Acid Amplification Test (e.g., PCR) on respiratory specimens, stool or rectal swabs, or blood.

Figure 3: Test for Coronavirus in the U.S.
- According to anecdotal reports, testing whole blood by PCR is more effective than testing plasma by PCR; thus, testing whole blood could be explored in patients with no etiology and who have tested negative for adenovirus in plasma samples.
- It is important to ensure that tests are carried out for both adenovirus and SARS-CoV-2 (PCR and serology), in addition to other infectious agents.
Sansure Adenovirus Nucleic Acid Test

Figure 4. ADV-Adenovirus Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing)
The Adenovirus Nucleic Acid Diagnostic Kit is used to assess for adenovirus nucleic acid in patients or close contacts who are considered to have adenovirus infections (e.g., cough, fever, dyspnea, bronchopneumonia, wheezing, lung infections, upper respiratory tract infections, etc.).
The findings can be used to aid in the diagnosis of adenovirus infection and provide a molecular diagnostic basis for adenovirus infection. An ideal diagnostic kit should be highly sensitive and should be quick and simple to operate with accurate results. Some specific features of the Sansure adenovirus nucleic acid test are described below, and this is why it should be preferred over other diagnostic kits:
Features of Sansure adenovirus nucleic acid test
- High sensitivity: Detection sensitivity reaches 200 copies/mL.
- Quick and easy: Perfectly match Sansure’s one-tube fast sample release reagent, easy to operate.
- IC monitoring: IC (Internal Control) monitoring test process to avoid false-negative results.
Sansure COVID-19 Nucleic Acid Test

Figure 5. Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing)
Sansure COVID-19 diagnostic kits can be used to precisely find the presence of viral RNA, which can be detected in patients before antibodies form or signs of the sickness appear, meaning the test results can tell whether or not someone is infected extremely early in their sickness. It has been observed during the COVID-19 pandemic how diagnostic and other healthcare centers struggled to detect a large number of viral samples.
An ideal COVID-19 diagnostic solution should be fast enough to screen out a large number of positive and negative samples. Below are some of the specific features which make it the best choice to test coronavirus:
Features of Sansure COVID-19 nucleic acid test
- One-tube/fast-release technology
- Up to 96 samples at one time
- Simple operation process, less specialist training
- Room temperature lysis, less contamination
- Enhance large-scale screening efficiency
For more information about How Sansure can help, please visit https://www.sansureglobal.com/.
References:
[1] Multi-Country – Acute, severe hepatitis of unknown origin in children.
Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON376 (Accessed: 1 August, 2022)
Note: The picture comes from the Internet. If there is any infringement, please contact the author to delete it.
Disclaimer: All the publications on this website, where the source is indicated, are copyrighted by the original source and do not represent the position of this website.