-


S3055E BP – Bordetella Pertussis DNA Diagnostic Kit
Respiratory Tract InfectionsBrief
Pertussis, also known as whooping cough, is a highly contagious respiratory infection caused by the bacterium Bordetella pertussis. Pertussis spreads easily from person to person mainly through droplets produced by coughing or sneezing. The disease is most dangerous in infants, and is a significant cause of disease and death in this age group.
Sansure kit is used to detect Bordetella pertussis DNA present in the nasopharyngeal swab specimens by applying PCR fluorescence probing technique. The detection result can be used as an aid in the diagnosis of bordetella pertussis.
Parameters
Product features Parameter Specimen Types Nasopharyngeal swabTechnical PlatformFast release technologyAdvanced magnetic beads technologyAnti-contamination systemUNG enzyme + dUTP systemInternal ControlInternal control plasmidPCR InstrumentsMx3000P; ABI 7500; MA-6000; S-Q31A/S-Q31B; S-Q36ASensitivity200 copies/mLObtained CertificatesNMPA, CE-IVDD etc. -


S3174E HCoV-MERS – Human Coronavirus (MERS) Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
The Human Coronavirus (MERS) is generally detectable in respiratory samples during the acute phase of infection. The Human Coronavirus (MERS) Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) can qualitatively detect HCoV-MERS in sputum, alveolar lavage fluid, and throat swabs, the test results can be used to assist in the diagnosis of patients infected with HCoV-MERS, providing a molecular diagnostic basis for coronavirus MERS infection. The test results of this kit are for clinical reference only, and should not be used as the sole criterion for clinical diagnosis. It is recommended to conduct a comprehensive analysis of the condition based on the patient's clinical manifestations and other laboratory tests.
Parameters
Product features Parameter Covering Genes MERS-CoV Orf1bSpecimen TypesSputum, alveolar lavage fluid, throat swabTechnical PlatformOne-tube fast release technologyAdvanced magnetic beads technologyInternal Control GeneRNase PCompatible InstrumentsABI 7500, Stratagene Mx3000P, SLAN®-96P, MA-6000, iPonatic S-Q36A/S-Q31A/S-Q31BSensitivity500 copies/mLQualificationCE -

S3353E FluA/Flu B – Influenza A/B Virus Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
Influenza Virus is a kind of RNA virus in the Orthomyxoviridae family which leading to human and animal influenza. It causes acute upper respiratory tract infection, spreads rapidly through the air and has periodic pandemics around the world. Human influenza virus are influenza pathogens which can be classified into three types, namely A, B and C. Among them, influenza A is the most harmful, while influenza B and influenza C have weak pathogenicity and are not easy to mutate.
The diagnostic Kit is intended for detection of the Influenza A and Influenza B in oropharyngeal swab from individuals. The test results can be used for the auxiliary diagnosis of respiratory Influenza A/B Virus infection and provide molecular diagnostic basis for Influenza A/B Virus infection.
Parameters
Product features Parameter Covering pathogensInfluenza A and Influenza BSpecimen TypesOropharyngeal swabTechnical PlatformOne-tube fast release technologyAdvanced magnetic beads technologyCompatible InstrumentABI 7500; MA-6000; SLAN®-96P; QuantGene 9600; iPonatic S-Q31A/S-Q31BSensitivity200 copies/mLSpec.48T, 24-PQualificationCE -


S3363E-12-P TB and RFP – Mycobacterium Tuberculosis Nucleic Acid and Rifampicin Resistance Fluorescence Diagnostic Kit
Respiratory Tract InfectionsBrief
Mycobacterium tuberculosis (M. tuberculosis) is the pathogen causing tuberculosis, which can invade all organs of the whole body, and pulmonary tuberculosis caused by pulmonary involvement is the most common. Early diagnosis and treatment are crucial measures to effectively control the spread of tuberculosis.
Due to the abuse of antibiotics or the insufficient course of drugs of patients, the sensitivity of patients to drugs weakens or even disappears, resulting in the decreasing or ineffective effect of drugs on pulmonary tuberculosis. According to the types of anti-tuberculosis drugs, drug-resistant tuberculosis can be divided into monoresistance pulmonary tuberculosis, polyresistance pulmonary tuberculosis, multidrug resistance pulmonary tuberculosis and extensively drug-resistant pulmonary tuberculosis. Rifampicin is one of the first-line drugs for the treatment of pulmonary tuberculosis.
The Mycobacterium Tuberculosis and Rifampicin Resistance Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) is a real-time polymerase chain reaction test kit intended for the qualitative detection of the nucleic acid of mycobacterium tuberculosis and rifampicin resistance mutations in human sputum samples. The test results can be used to assist in the diagnosis of TB patients and patients with an increased risk of RFP drug-resistant TB, providing a molecular diagnosis basis for infected patients.
Parameters
The kit is registered in Indonesia.Product features Parameter Specimen Type Sputum Technical PlatformOne-tube fast release technologyPCR InstrumentsiPonatic Ⅲ (S-Q36A)Internal ControlRNase PLimit of detectionMycobacterium tuberculosis 1,000 Bacteria/mL;Rifampicin resistance 10,000 Bacteria/mL -

S3016E MP – Mycoplasma Pneumoniae DNA Fluorescence Diagnostic Kit
Respiratory Tract InfectionsBrief
Mycoplasma pneumoniae (MP) is a pathogenic microorganism between bacteria and virus. It is mainly transmitted through buccal and nasal mucus by the air causing respiratory diseases, with the highest incidence in children and adolescents. Respiratory infection has the manifestations of pharyngitis and bronchitis, with a few cases causing infection to the lung. Recently, incidence among infants and children is increasing, therefore, early diagnosis and treatment can decrease the exacerbation of acute pneumonia in children. The development of molecular biology also draws more attention to the fluorescence quantitative PCR technology for the detection of MP-DNA. This diagnostic kit is an in vitro nucleic acid amplification test for the detection of mycoplasma pneumoniae DNA in humansputum and throat swab. It is intended for use as an aid in the diagnosis of an MP infection and providing a molecular-diagnostics-based solution.Parameters
Product features Parameter Specimen Type Sputum and throat swab Extraction Platform One-tube fast release technology Internal Control cloning plasmid containing the target gene fragment PCR Instrument ABI 7500, SLAN-96P,MA-6000, Roche LightCycler 480, iPonatic S-Q31A&B,S-Q36A Sensitivity 400 copies/mL Spec. 48T, 12-P Qualification CE -


S3066E 6RP – Six Respiratory Pathogens Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
Respiratory infections are classified into the upper respiratory tract infections and lower respiratory tract infections. It refer to the pathogens that infect the nose, throat, trachea, bronchi or lungs, which principally cause diseases of tissues and organs outside the respiratory tract, manifested by fever, sore throat, cough, headache and other symptoms. The respiratory tract pathogen has the characteristics of strong infectivity, rapid spread, short incubation period and acute onset, etc. which seriously harm human health. After respiratory infection, symptoms are mostly similar. Sansure six respiratory pathogens joint detection kits can help doctors make differential diagnosis, accurately detect the pathogens that cause symptoms, and formulate treatment plans.Performance
- High sensitivity: Super-cis-nanometer magnetic bead technology; can achieve 500copies/mL
- Accurate identification: One test presented six results ; accurate guidance for rational clinical drug use
- Whole-process monitoring: Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) human housekeeping gene as internal standard; Monitor the whole process of sampling, nucleic acid extraction and amplification
Parameters
Product features Parameter Specimen Type Nasopharyngeal swabs Extraction Platform Advanced magnetic beads technology Internal Control lentivirus particles(GAPDH) PCR Instrument SLAN-96P, ABI7500, S-Q36A Sensitivity Influenza A virus: 2.0 TCID50/mL Influenza B virus: 2.0 TCID50/mL Respiratory syncytial virus: 500.0 copies/mL Adenovirus: 500.0 copies/mL Mycoplasma pneumonia: 500.0 copies/mL Human rhinovirus: 500.0 copies/mL Spec. 24T, 12P Qualification CE -

S3334E AdV – Adenovirus Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
Acute infectious disease caused by adenovirus, easily affects the mucous membranes of the respiratory and digestive tracts, the conjunctiva of the eyes, the urinary tract and the lymph nodes. The main manifestation is an acute upper respiratory tract infection. The population is generally susceptible, mostly the children. Infants are susceptible to adenovirus pneumonia, which is severe and has a high mortality rate. The source of infection is the patient and the latent infected person. The virus is excreted from the respiratory tract and conjunctival secretions, feces and urine, and is transmitted by airborne droplets, close contact and the feces-oral route. The Adenovirus Nucleic Acid Diagnostic Kit is used for nucleic acid testing of adenovirus in patients suspected of being adenovirus infections (e.g., fever, cough, wheezing, dyspnea, bronchopneumonia, upper respiratory tract infections, lung infections, etc.) or related close contacts, and the results can be used to assist in the diagnosis of adenovirus infection and provide a molecular diagnostic basis for adenovirus infection.Features
- High sensitivity: Detection sensitivity reaches 200 copies/mL.
- Quick and easy: Perfectly match Sansure's one-tube fast Sample Release Reagent, easy to operate.
- IC monitoring: IC(Internal Control) monitoring test process to avoid false negative results.
Parameters
Items Parameter Specimen Type Throat swab Extraction Platform One-tube fast release technology Advanced magnetic beads technology Anti-contamination system UNG enzyme + dUTP system PCR Instrument ABI 7500; MA-6000; SLAN-96P; QuantStudio 5; iPonatic S-Q31A&B; S-Q36A Sensitivity 200 copies/mL Qualification NMPA, CE -


S3018E TB – Mycobacterium Tuberculosis DNA Fluorescence Diagnostic Kit
Respiratory Tract InfectionsBrief
Mycobacterium tuberculosis virus (TB) is a pathogenic bacterium that causes tuberculosis. It is likely to infect all human tissues and organs, especially the lungs to cause pulmonary tuberculosis. Early diagnosis and treatment are important for effective control of tuberculosis. In recent years, with the development of molecular biology, nucleic acid fluorescence quantitative PCR method based on the mycobacterium tuberculosis nucleic acid has drawn more and more attention from researchers.Parameters
Product features Parameter Specimen Type Human sputum Extraction Platform One-tube fast release technology Internal Control Cloning plasmid, without TB target sequence PCR Instrument ABI 7500, SLAN-96P,MA-6000, Stratagene Mx3000P, iPonatic S-Q31A&B,S-Q36A Sensitivity 1 bacterium/mL Spec. 48T, 12-P Qualification CE -


S3310E 6LRP – Six Respiratory Pathogens Multiplex Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
Lower respiratory infections (LRP) remained the world’s most deadly communicable disease, ranked as the 4th leading cause of death. In 2019 it claimed 2.6 million lives. Diseases of the lower respiratory tract include acute tracheitis, bronchitis, pneumonia, chronic bronchitis, chronic obstructive pulmonary disease, bronchiectasis, etc. They are caused by microbial infections such as viruses, bacteria, mycoplasma, chlamydia and legionella. Bacteria are the main pathogens of lower respiratory tract infections, with a wide variety of pathogens and complex clinical presentations. Due to the long detection period and low positive detection rate of traditional pathogenic tests, over 62% of adults with community-acquired pneumonia have no pathogenic basis. Failure to identify the cause quickly can delay treatment, exacerbate the disease and lead to death, and increase the development of antibiotic resistance.Features
- Highly efficient identification and rapid diagnosis: Six common bacteria of lower respiratory tract infections can be detected in one tube; a batch of 94 sample tests can finish in 100 minutes.
- Accurate and reliable, high detection rate: sensitive and specific, unaffected by antibacterial drugs, full internal control monitoring to avoid false negatives, UDG enzyme + dUTP anti- contamination measures to reduce false positives.
- Easy to operate and adaptable: automatic instruments are available, and the results can be intelligently analyzed by conventional fluorescent PCR instruments to meet the needs of medical laboratories , clinical Institutions, emergency and primary care etc.
Parameters
Items Parameter Specimen Type Sputum Extraction Platform Advanced magnetic beads technology Internal Control Plasmid PCR Instrument Thermofisher QuantStudio™ 5 and SLAN-96P Sensitivity 15 CFU/mL (Streptococcus pneumoniae) 340 CFU/mL (Legionella pneumophila) 625 CFU/mL (Haemophilus influenzae) 675 CFU/mL (Pseudomonas aeruginosa) 900 CFU/mL (Klebsiella pneumoniae) 2875 CFU/mL (Staphylococcus aureus) Qualification NMPA, CE -
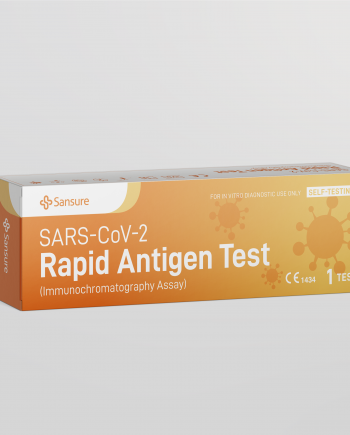
S3120E-H-SARS-CoV-2 Rapid Antigen Test for Self-testing
Respiratory Tract InfectionsBrief
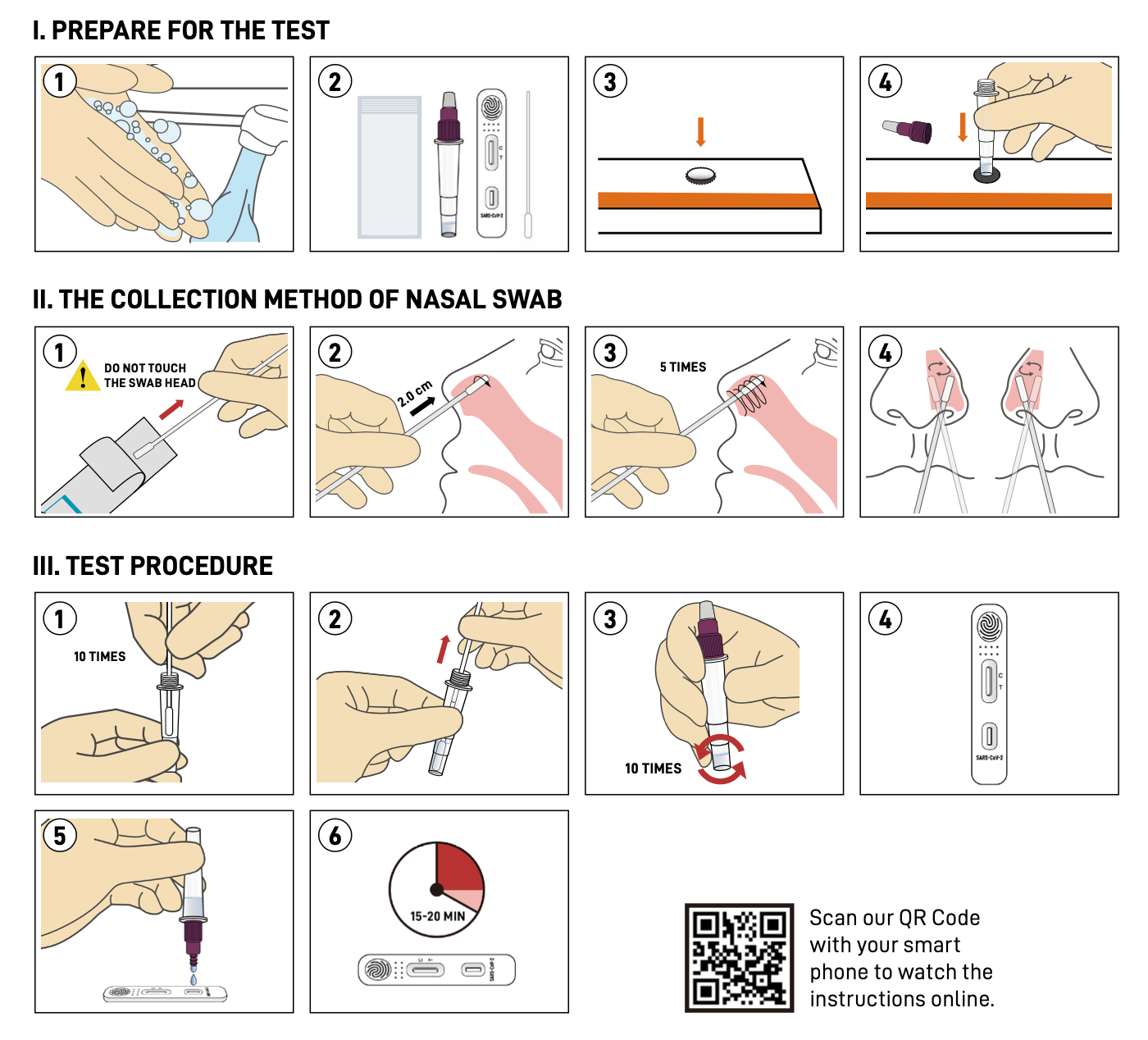
The SARS-CoV-2 Rapid Antigen Test (Immunochromatography Assay) for self-testing is authorized for home use with self-collected nasal swab samples to directly detect antigen of SARS-CoV-2 virus. With the help of this kit, people without professional training can also easily acquire their COVID-19 test result within 15-20 minutes.Features
- Accurate Sensitivity 94.55%, Specificity 100%
- Convenient No instrument needed, easy to operate
- Fast Result available in only 15-20 min
- Flexible Get tested anytime when you are free
Instruction
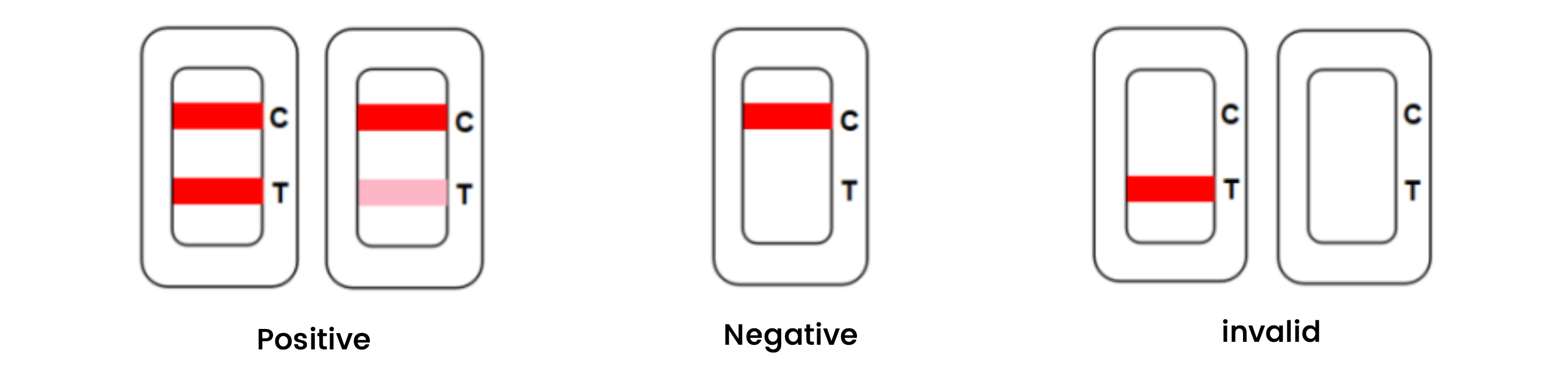
Result interpretation
Parameters
Qualification CE Kit components
No. Product Name Spec. S3120E-1-H, S3120E-5-H SARS-CoV-2 Rapid Antigen Test (Immunochromatography Assay) 1 Test, 5 Tests The kit components:
- SARS-CoV-2-Antigen Test Cassette (with desiccant)
- SARS-CoV-2 Sample Extraction Buffer
- Swab
- Plastic Waste Bag
-
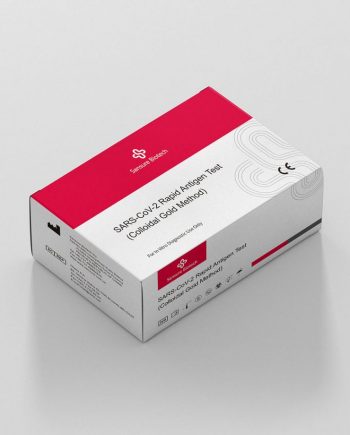

S3109E SARS-CoV-2 Rapid Antigen Test (Colloidal Gold Method)
Respiratory Tract InfectionsBrief
The SARS-CoV-2 Rapid Antigen Test (Colloidal Gold Method) is intended for the qualitative detection of the SARS-CoV-2 nucleocapsid protein in human nasopharyngeal or nasal swab sampled from individuals suspected of COVID-19.Parameters
Testing Time 10-15 minutes Sensitivity 98.4% Specificity 98.1% Qualification CE Kit components
No. Product Name Spec. S3109E-25 SARS-CoV-2 Rapid Antigen Test (Colloidal Gold Method) 25 tests -


S3113E SC2/FluA/B – SARS-CoV-2 and Influenza A/B Virus Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
Most people infected with the COVID-19 virus will experience mild to moderate respiratory illness and recover without requiring special treatment. Elder people, and those with underlying medical problems like cardiovascular disease, diabetes, chronic respiratory disease, and cancer are more likely to develop serious illness. Sansure COVID-19 diagnostic solutions are used to directly detect the presence of viral RNA, which will be detectable in patients before antibodies form or symptoms of the disease are present, which means the test results can tell whether or not that someone gets virus very early on in their illness. This kit can also joint-detect RNA of influenza A virus and influenza B virus.Parameters
Product features Parameters Specimen Type Oropharyngeal swab, sputum Extraction Platform One-tube fast release technology Advanced magnetic beads technology Target Genes SARS-CoV-2:ORF 1ab, N gene; influenza A:M gene; influenza B:NP gene Internal Control Rnase P gene PCR Instrument ABI7500, SLAN-96P, MA-6000, QuantGene 9600, iPonatic S-Q31A&B,S-Q36A Sensitivity 200 copies/mL Spec. 24T, 48T, 24-P Qualification CE -

S3148E SC2/Flu/RSV – SARS-CoV-2, Influenza Virus and Respiratory Syncytial Virus Multiple Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
The SARS-CoV-2, Influenza Virus and Respiratory Syncytial Virus Multiple Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) is a real-time RT-PCR test intended for the qualitative Diagnostic of nucleic acid from SARS-CoV-2, Influenza Virus and Respiratory Syncytial Virus Multiple in the nasopharyngeal swabs and oropharyngeal swabs from individuals.
As the seventh coronavirus that infects humans, the SARS-CoV-2 can cause fever, fatigue, dry cough, dyspnea and other symptoms. In severe cases, it can cause acute respiratory distress syndrome, septic shock, and even death. At the same time, the SARS-CoV-2 has a strong spreading ability and has a wide range. Influenza virus (Influenza virus) can cause acute respiratory infections, with clinical manifestations of fever, headache, myalgia, fatigue, rhinitis, sore throat and cough. Influenza viruses can aggravate underlying diseases (such as heart and lung diseases) or cause secondary bacterial pneumonia or primary influenza viral pneumonia. The elderly and people with various chronic diseases or physical weakness are prone to severe complications and mortality higher after infecting influenza. Respiratory syncytial virus (RSV) belongs to the Pneumovirus genus of the Paramyxoviridae family. It mainly causes lower respiratory tract infections such as bronchiolitis and pneumonia in infants under 6 months, as well as rhinitis , Cold and other upper respiratory tract infections in older children and adults.
Parameters
Product features Parameter Covering pathogensSARS-CoV-2, Influenza Virus and Respiratory Syncytial VirusSpecimen TypesNasopharyngeal swab and oropharyngeal swabTechnical PlatformOne-tube fast release technologyAdvanced magnetic beads technologyInternal ControlRNase P geneCompatible InstrumentABI 7500; MA-6000; SLAN®-96P; iPonatic S-Q31A/S-Q31B/S-Q36ASensitivity500 copies/mL.QualificationCE -


S3102E SC2 – Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
COVID-19 is an infectious disease caused by a newly discovered coronavirus named SARS-CoV2. Most people infected with the COVID-19 virus will experience mild to moderate respiratory illness and recover without requiring special treatment. Elder people, and those with underlying medical problems like cardiovascular disease, diabetes, chronic respiratory disease, and cancer are more likely to develop serious illness. Sansure COVID-19 diagnostic solutions are used to directly detect the presence of viral RNA, which will be detectable in patients before antibodies form or symptoms of the disease are present, which means the test results can tell whether or not that someone gets virus very early on in their illness.Performance
- One-tube/fast release technology
- Up to 96 samples at one time
- Simple operation process, less specialist training
- Room temperature lysis, less contamination
- Sampling types : nasopharyngeal swab, oropharyngeal swab, alveolar lavage fluid, sputum, serum, whole blood ,Feces
- Enhance large-scale screening efficiency
- Internal control: human housekeeping gene RNase P
Parameters
Product features Parameters Specimen Type Nasopharyngeal swab, oropharyngeal swab, alveolar lavage fluid, sputum, serum, whole blood, feces Extraction Platform One-tube fast release technology Advanced magnetic beads technology Internal Control Rnase P gene PCR Instrument ABI7500, QuantStudioTM 5, SLAN-96P, MA-6000, Bio-Rad CFX-96, QuantGene 9600, LightCycler 480, iPonatic S-Q31A&B,S-Q36A Sensitivity 200 copies/mL Spec. 24T, 48T, 24-P Qualification CE -

S3121E SARS-CoV-2/InFluA/InFluB Rapid Antigen Test (Immunochromatography Assay)
Respiratory Tract InfectionsBrief
SARS-CoV-2/InFluA/InFluB Rapid Antigen Test (Immunochromatography Assay) is intended for the qualitative detection of the SARS-CoV-2/InFluA/InFluB nucleocapsid protein in human nasopharyngeal or oropharyngeal swabs. Test results will be available for reading in 15-20 minutes. Positive results indicate the presence of viral antigens. This kit is for in vitro diagnostic use.
Features
- Accurate LOD 80TCID50 /ml, Specificity 100%
- Convenient No instrument needed, easy to operate
- Fast Result available in only 15-20 min
Instruction
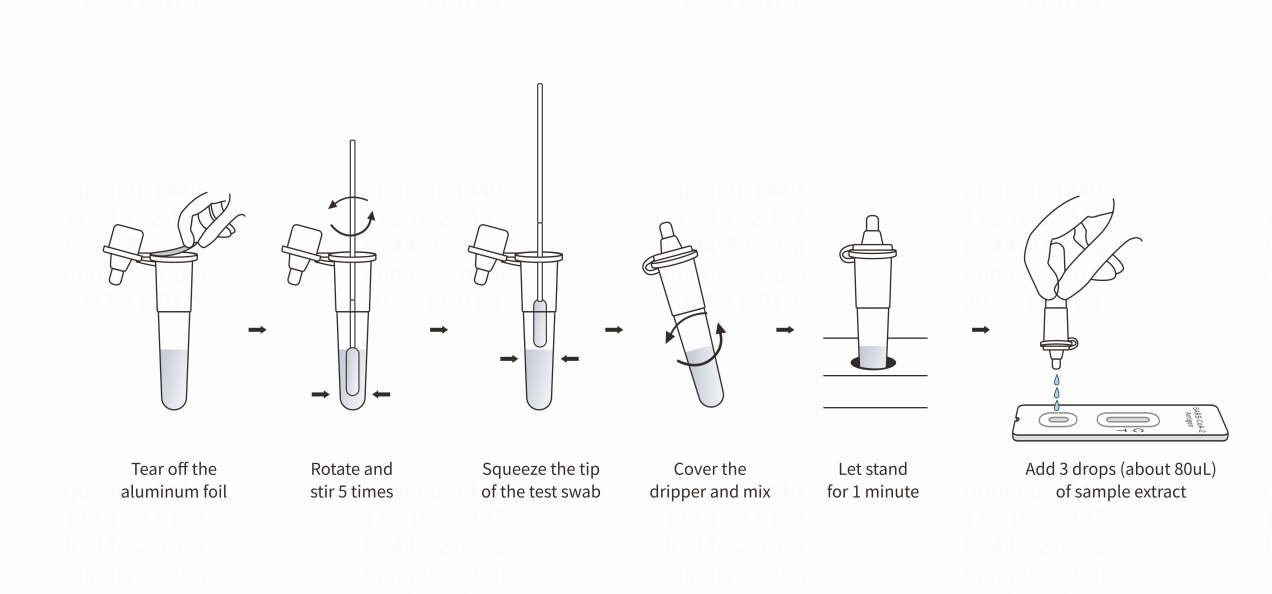
Result interpretation
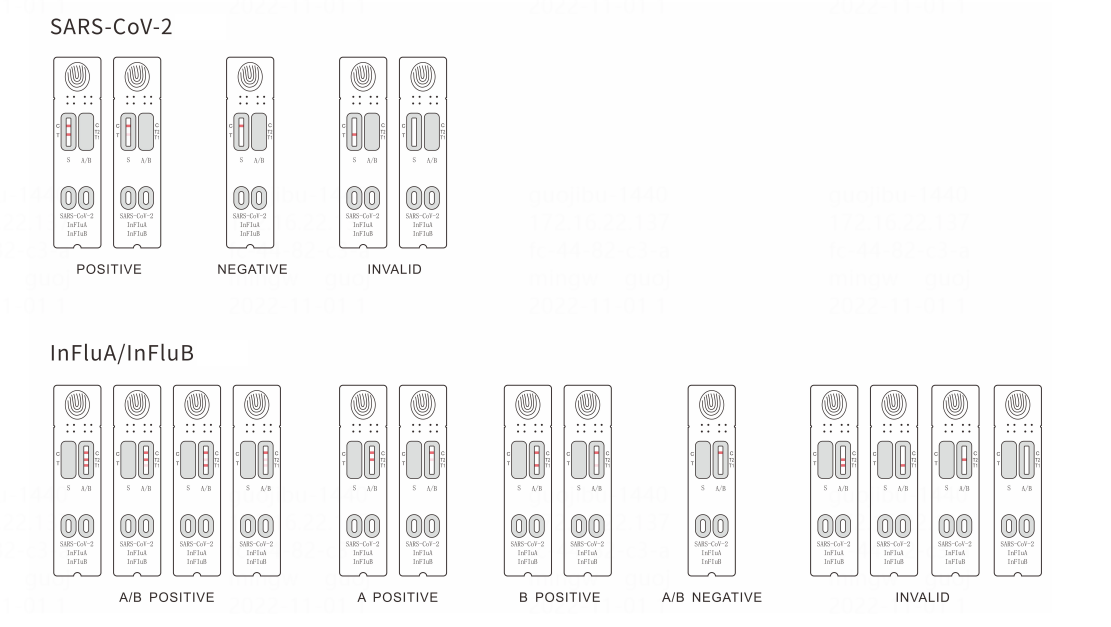
Parameters
Qualification CE Kit components
No. Product Name Spec. S3121E-25SARS-CoV-2/InFluA/InFluB Rapid Antigen Test (Immunochromatography Assay)25 Test The kit components:
- SARS-CoV-2/InFluA/InFluB Antigen Test Cassette (individually in a foil pouch with desiccant)
- Sample Extraction Buffer
- Swab
