The epidemic in Hong Kong has touched people’s hearts. With the sincere care for our compatriots in Hong Kong, on the morning of April 20, Sansure Biotech donated HK$6.3 million of Covid-19 antigen tests to the channel of Chinese Culture Foundation and Hunan Charity Federation for the prevention and control of the epidemic in Hong Kong schools, helping Hong Kong […]
On April 13, the SARS-CoV-2 Rapid Antigen Test (Self-testing) developed by Sansure Biotech has obtained the CE 1434 certificate, which means that the self-test kit can be distributed in EU countries and countries that recognize the EU CE certification. SARS-CoV-2 antigen test is an important means of epidemic prevention and control, which can be used as an auxiliary diagnosing method of […]
The Respiratory Adenovirus DNA Diagnostic Kit (PCR-Fluorescence Probing) developed by Sansure was approved for marketing lately, adding another powerful tool to its total solution of respiratory nucleic acid test. Adenovirus, an easily neglected but dangerous respiratory infection pathogen Why is it dangerous and easily neglected? This is because adenovirus is also a virus that is strongly associated with respiratory diseases and […]
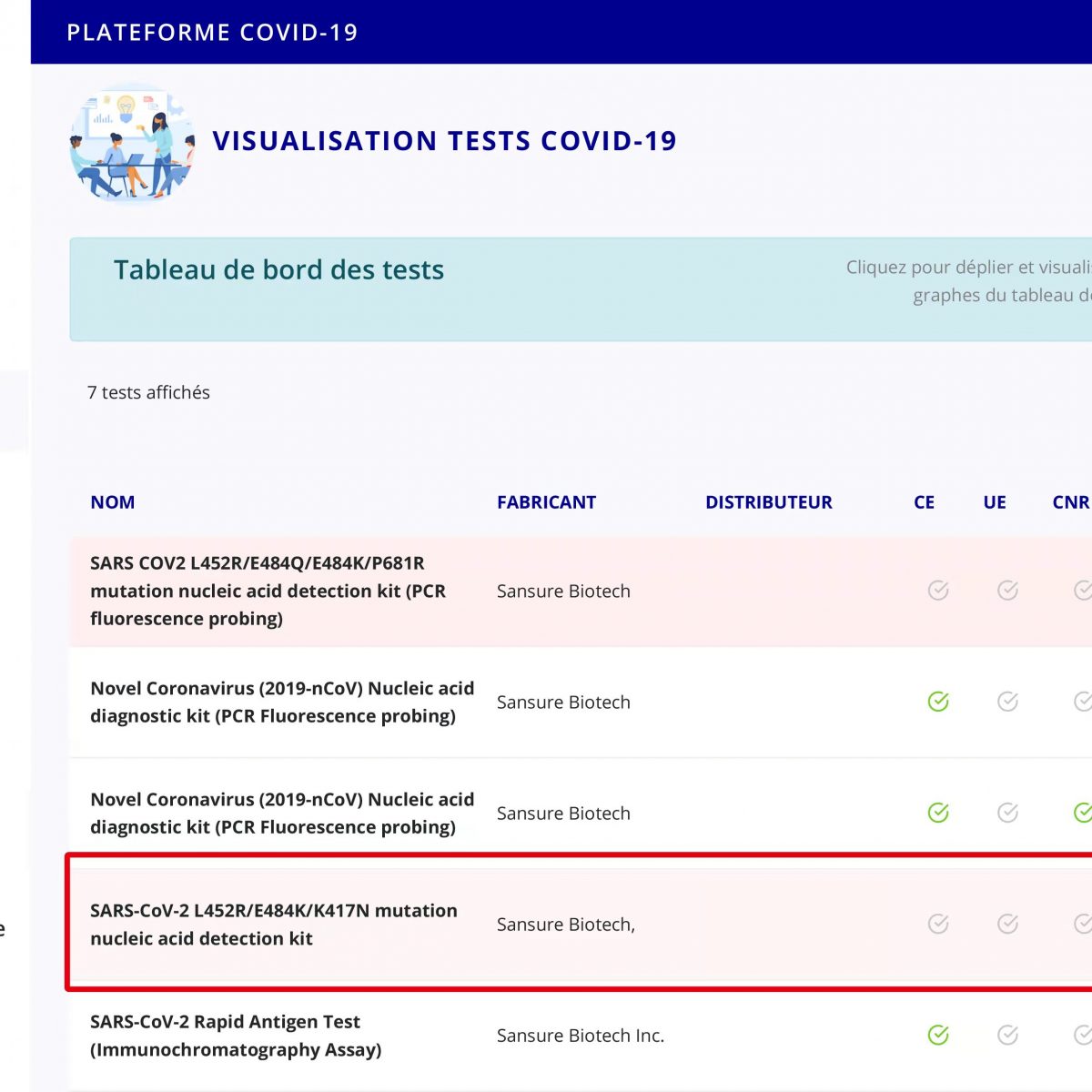
Sansure Biotech’s SARS-CoV-2 L452R/E484K/K417N Variant Diagnostic Kit received marketing authorization approval from Ministère des Solidarités et de la Santé (Ministry of Solidarity and Health) the other day. This is Sansure Biotech’s fifth SARS-CoV-2 detection product that has received marketing authorization approval from Ministère des Solidarités et de la Santé. The Omicron variant of SARS-CoV-2 is now spreading rapidly and rampantly […]
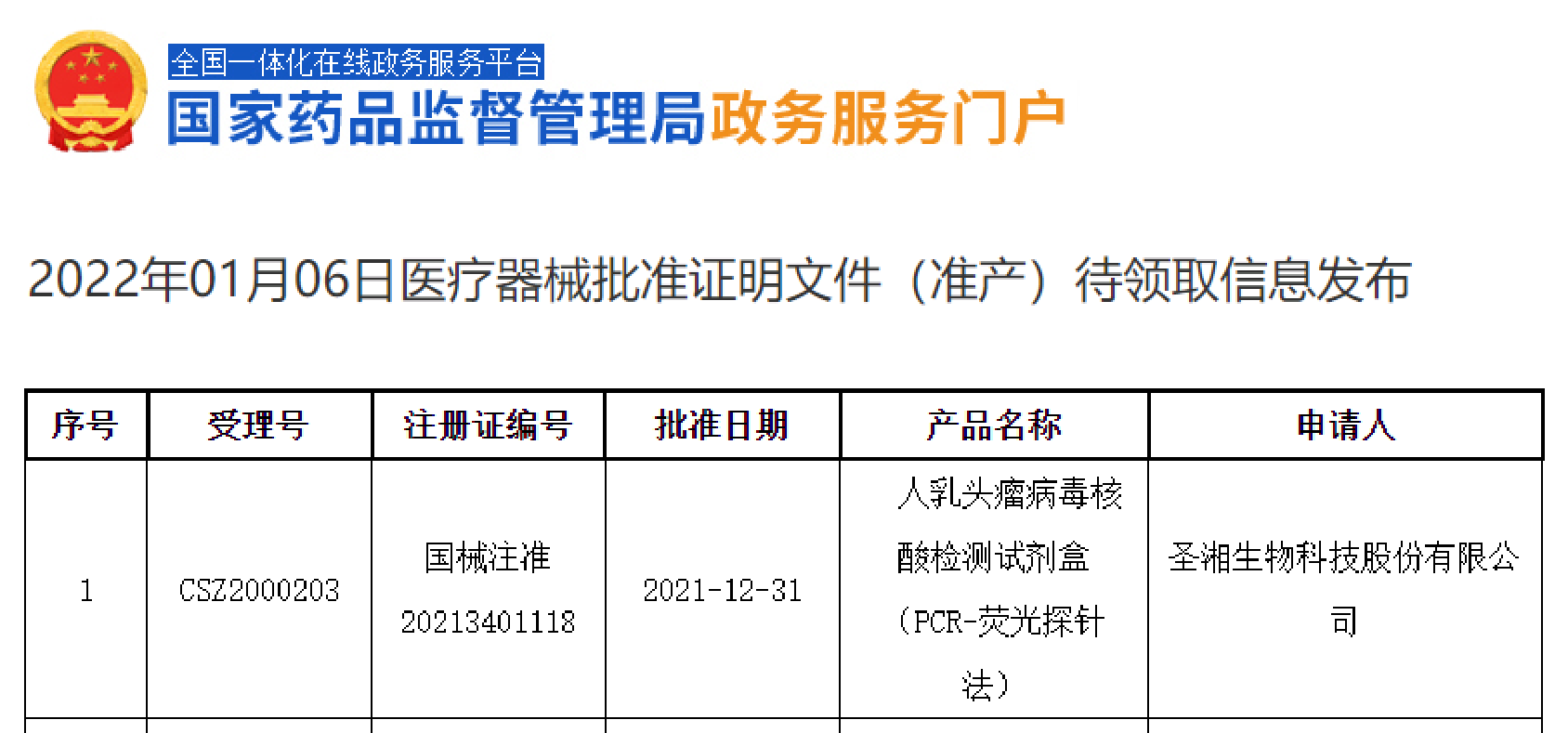
Sansure Biotech’s HPV DNA Diagnostic Kit (HPV 13+2) was approved by the China NMPA on January 6, with reference to the medical device approval released on the NMPA’s official website. This is another blockbuster product from Sansure Biotech in promoting the global elimination of cervical cancer. In 2020, WHO launched a global strategy to accelerate the elimination of cervical cancer, […]