-

S3121E SARS-CoV-2/InFluA/InFluB Rapid Antigen Test (Immunochromatography Assay)
Respiratory Tract InfectionsBrief
SARS-CoV-2/InFluA/InFluB Rapid Antigen Test (Immunochromatography Assay) is intended for the qualitative detection of the SARS-CoV-2/InFluA/InFluB nucleocapsid protein in human nasopharyngeal or oropharyngeal swabs. Test results will be available for reading in 15-20 minutes. Positive results indicate the presence of viral antigens. This kit is for in vitro diagnostic use.
Features
- Accurate LOD 80TCID50 /ml, Specificity 100%
- Convenient No instrument needed, easy to operate
- Fast Result available in only 15-20 min
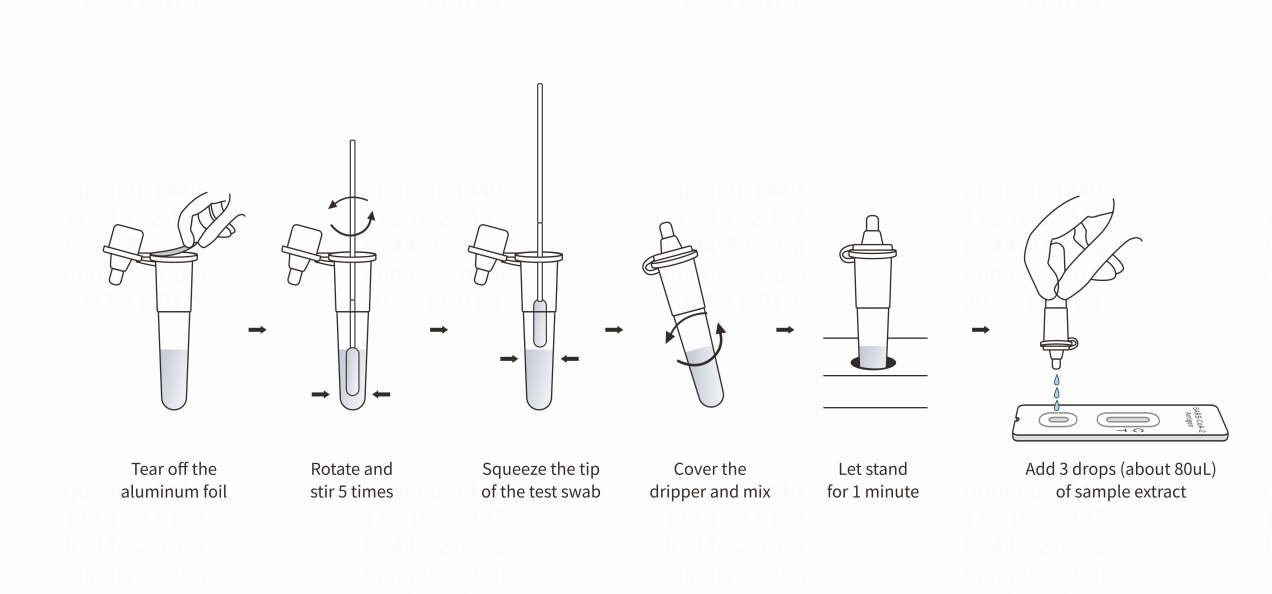
Instruction
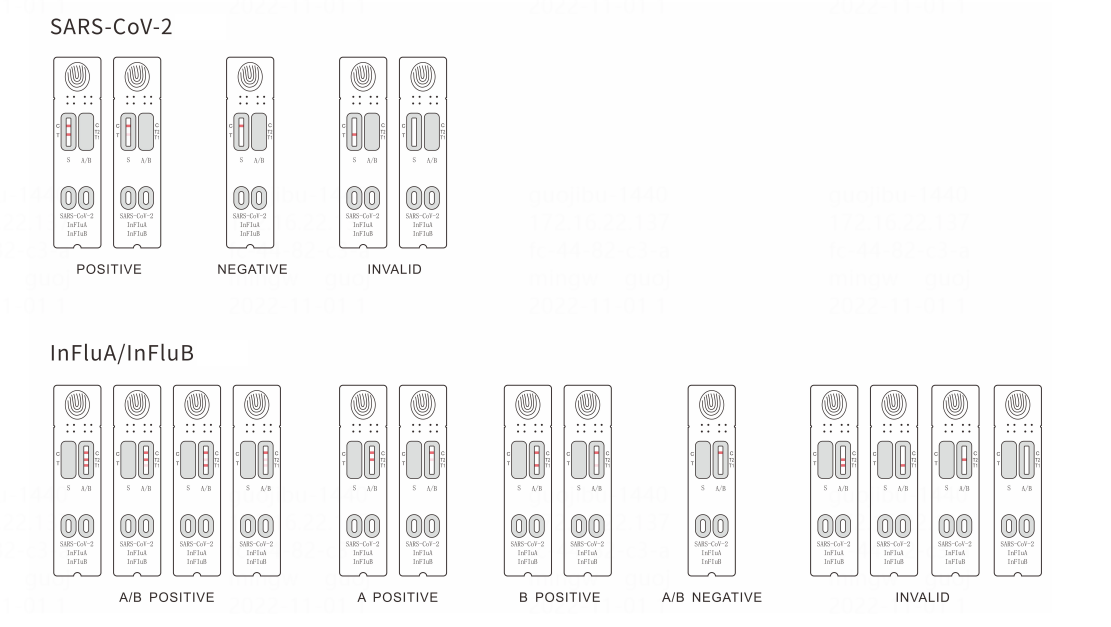
Result interpretation
Parameters
Qualification CE Kit components
No. Product Name Spec. S3121E-25SARS-CoV-2/InFluA/InFluB Rapid Antigen Test (Immunochromatography Assay)25 Test The kit components:
- SARS-CoV-2/InFluA/InFluB Antigen Test Cassette (individually in a foil pouch with desiccant)
- Sample Extraction Buffer
- Swab
