-


S3004E UU – Ureaplasma Urealyticum DNA Fluorescence Diagnostic Kit
Reproductive Tract InfectionsBrief
The Ureaplasma Urealyticum (UU) DNA Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is used to detect the presence of UU-DNA in samples such as male urethra swab and female cervical swab. The detection result can be used as an aid in the diagnosis of a UU infection. The test provides a molecular diagnostics-based solution for early diagnosis of venereal disease and for preliminary screening of high-risk venereal disease groups.Parameters
Product features Parameter Specimen Type Genital tract secretions. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen UU Internal Control Recombinant plasmid PCR Instrument ABI7500,QuantStudio 5, Stratagene Mx3000P, MA-6000, SLAN-96P, LightCycler® 480 II,iPonatic S-Q31A&B,S-Q36A Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T, 12-P Qualification CE -


S3003E NG – Neisseria Gonorrhoeae DNA Fluorescence Diagnostic Kit
Reproductive Tract InfectionsBrief
Neisseria Gonorrhoeae (NG) DNA Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) is used to detect the presence of NG-DNA in samples such as male urethral swab and female cervical swab. The detection result can be used as an aid in the diagnosis of an NG infection. The test provides a molecular diagnostics-based solution for early diagnosis of venereal disease and for preliminary screening of high-risk venereal disease groups.Parameters
Product features Parameter Specimen Type Genital tract secretions. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen NG Internal Control Recombinant plasmid PCR Instrument ABI7500, QuantStudio 5, Stratagene Mx3000P, MA-6000, SLAN-96P, LightCycler® 480 II, iPonatic S-Q31A&B, S-Q36A Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T, 12-P Qualification CE -

S3001E CT – Chlamydia Trachomatis DNA Fluorescence Diagnostic Kit
Reproductive Tract InfectionsBrief
Chlamydia Trachomatis (CT) DNA Fluorescence Diagnostic Kit (PCR-Fluorescence Probing) applies to detect CT DNA in specimens like reproductive tract secretion. The detection result can be used for aiding diagnosis of CT infection, which provides molecular diagnosis base for early diagnosis of venereal disease and for preliminary screening of venereal disease high-risk groups.Parameters
Product features Parameter Specimen Type genital tract secretions. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen CT Internal Control Recombinant plasmid PCR Instrument ABI 7500, Roche LC480,Stratagene Mx3000P Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T Qualification CE -


S3050E CT/UU/NG – Chlamydia Trachomatis/Ureaplasma Urealyticum/Neisseria Gonorrhoeae DNA Diagnostic Kit
Reproductive Tract InfectionsBrief
Chlamydia Trachomatis/Ureaplasma Urealyticum/Neisseria Gonorrhoeae DNA Diagnostic Kit (PCR-Fluorescence Probing) is a qualitative in vitro test for simultaneous detection of Chlamydia Trachomatis, Ureaplasma Urealyticum and Neisseria Gonorrhoeae DNA in sterile calcium alginate swab specimens from male urinary tract secretion and female genital tract secretion by applying real-time fluorescence quantitative PCR technique, which can provide molecular diagnosis evidence for the early diagnosis of related sexually transmitted diseases and initial screening of high-risk STD population.Parameters
Product features Parameters Specimen Type genital tract secretions. Technical Platform One-tube fast release technology Advanced magnetic beads technology Target pathogen CT, UU, NG-DNA Internal Control β--globin gene PCR Instruments ABI7500,SLAN-96P Amplification Time 70 min Sensitivity 400 copies/mL Spec. 48T Qualification CE -


S3113E SC2/FluA/B – SARS-CoV-2 and Influenza A/B Virus Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
Most people infected with the COVID-19 virus will experience mild to moderate respiratory illness and recover without requiring special treatment. Elder people, and those with underlying medical problems like cardiovascular disease, diabetes, chronic respiratory disease, and cancer are more likely to develop serious illness. Sansure COVID-19 diagnostic solutions are used to directly detect the presence of viral RNA, which will be detectable in patients before antibodies form or symptoms of the disease are present, which means the test results can tell whether or not that someone gets virus very early on in their illness. This kit can also joint-detect RNA of influenza A virus and influenza B virus.Parameters
Product features Parameters Specimen Type Oropharyngeal swab, sputum Extraction Platform One-tube fast release technology Advanced magnetic beads technology Target Genes SARS-CoV-2:ORF 1ab, N gene; influenza A:M gene; influenza B:NP gene Internal Control Rnase P gene PCR Instrument ABI7500, SLAN-96P, MA-6000, QuantGene 9600, iPonatic S-Q31A&B,S-Q36A Sensitivity 200 copies/mL Spec. 24T, 48T, 24-P Qualification CE -

S3148E SC2/Flu/RSV – SARS-CoV-2, Influenza Virus and Respiratory Syncytial Virus Multiple Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
The SARS-CoV-2, Influenza Virus and Respiratory Syncytial Virus Multiple Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) is a real-time RT-PCR test intended for the qualitative Diagnostic of nucleic acid from SARS-CoV-2, Influenza Virus and Respiratory Syncytial Virus Multiple in the nasopharyngeal swabs and oropharyngeal swabs from individuals.
As the seventh coronavirus that infects humans, the SARS-CoV-2 can cause fever, fatigue, dry cough, dyspnea and other symptoms. In severe cases, it can cause acute respiratory distress syndrome, septic shock, and even death. At the same time, the SARS-CoV-2 has a strong spreading ability and has a wide range. Influenza virus (Influenza virus) can cause acute respiratory infections, with clinical manifestations of fever, headache, myalgia, fatigue, rhinitis, sore throat and cough. Influenza viruses can aggravate underlying diseases (such as heart and lung diseases) or cause secondary bacterial pneumonia or primary influenza viral pneumonia. The elderly and people with various chronic diseases or physical weakness are prone to severe complications and mortality higher after infecting influenza. Respiratory syncytial virus (RSV) belongs to the Pneumovirus genus of the Paramyxoviridae family. It mainly causes lower respiratory tract infections such as bronchiolitis and pneumonia in infants under 6 months, as well as rhinitis , Cold and other upper respiratory tract infections in older children and adults.
Parameters
Product features Parameter Covering pathogensSARS-CoV-2, Influenza Virus and Respiratory Syncytial VirusSpecimen TypesNasopharyngeal swab and oropharyngeal swabTechnical PlatformOne-tube fast release technologyAdvanced magnetic beads technologyInternal ControlRNase P geneCompatible InstrumentABI 7500; MA-6000; SLAN®-96P; iPonatic S-Q31A/S-Q31B/S-Q36ASensitivity500 copies/mL.QualificationCE -


S3102E SC2 – Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit
Respiratory Tract InfectionsBrief
COVID-19 is an infectious disease caused by a newly discovered coronavirus named SARS-CoV2. Most people infected with the COVID-19 virus will experience mild to moderate respiratory illness and recover without requiring special treatment. Elder people, and those with underlying medical problems like cardiovascular disease, diabetes, chronic respiratory disease, and cancer are more likely to develop serious illness. Sansure COVID-19 diagnostic solutions are used to directly detect the presence of viral RNA, which will be detectable in patients before antibodies form or symptoms of the disease are present, which means the test results can tell whether or not that someone gets virus very early on in their illness.Performance
- One-tube/fast release technology
- Up to 96 samples at one time
- Simple operation process, less specialist training
- Room temperature lysis, less contamination
- Sampling types : nasopharyngeal swab, oropharyngeal swab, alveolar lavage fluid, sputum, serum, whole blood ,Feces
- Enhance large-scale screening efficiency
- Internal control: human housekeeping gene RNase P
Parameters
Product features Parameters Specimen Type Nasopharyngeal swab, oropharyngeal swab, alveolar lavage fluid, sputum, serum, whole blood, feces Extraction Platform One-tube fast release technology Advanced magnetic beads technology Internal Control Rnase P gene PCR Instrument ABI7500, QuantStudioTM 5, SLAN-96P, MA-6000, Bio-Rad CFX-96, QuantGene 9600, LightCycler 480, iPonatic S-Q31A&B,S-Q36A Sensitivity 200 copies/mL Spec. 24T, 48T, 24-P Qualification CE -

S3121E SARS-CoV-2/InFluA/InFluB Rapid Antigen Test (Immunochromatography Assay)
Respiratory Tract InfectionsBrief
SARS-CoV-2/InFluA/InFluB Rapid Antigen Test (Immunochromatography Assay) is intended for the qualitative detection of the SARS-CoV-2/InFluA/InFluB nucleocapsid protein in human nasopharyngeal or oropharyngeal swabs. Test results will be available for reading in 15-20 minutes. Positive results indicate the presence of viral antigens. This kit is for in vitro diagnostic use.
Features
- Accurate LOD 80TCID50 /ml, Specificity 100%
- Convenient No instrument needed, easy to operate
- Fast Result available in only 15-20 min
Instruction
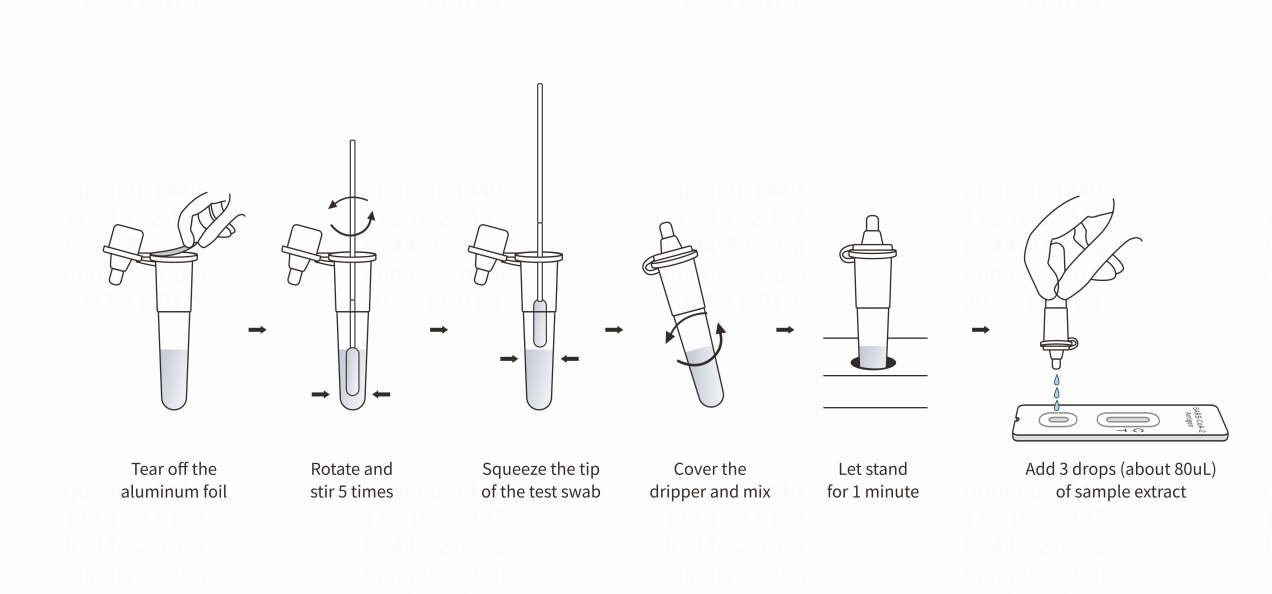
Result interpretation
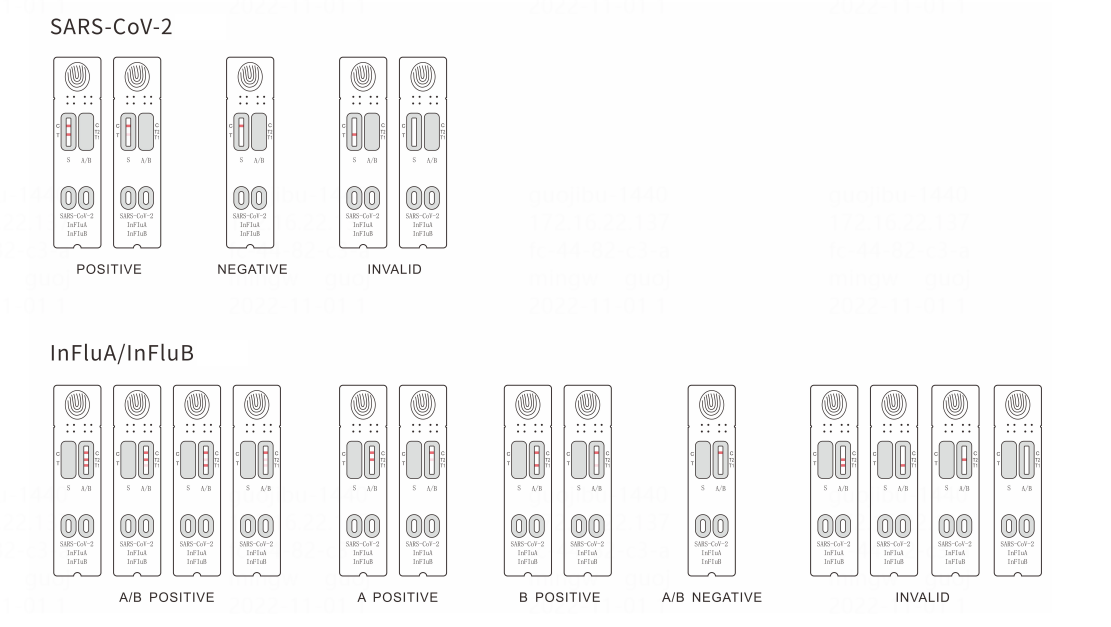
Parameters
Qualification CE Kit components
No. Product Name Spec. S3121E-25SARS-CoV-2/InFluA/InFluB Rapid Antigen Test (Immunochromatography Assay)25 Test The kit components:
- SARS-CoV-2/InFluA/InFluB Antigen Test Cassette (individually in a foil pouch with desiccant)
- Sample Extraction Buffer
- Swab
