iPonatic — Portable Molecule Workstation
Brief
Innovations in molecular assays—especially on the point-of-care testing (POCT) front—have spread this testing from molecular diagnostics laboratories into clinical microbiology laboratories—and now into general laboratories and even clinics and exam rooms.[1]
Access to sensitive and rapid infectious disease diagnostic assays is essential for accurate diagnosis, effective treatment, and timely infection control, making POCT vital to reducing TAT. With novel POCT on the horizon, future studies are warranted to determine cost savings, antimicrobial usage, TAT, patient impact, and how to best implement in non-microbiology clinical laboratories and clinics.[2]
Sansure iPonatic (Portable Molecule Workstation) aims to innovate traditional diagnostic mode and assist precision diagnosis. It can provide quickly and convenient diagnosis experience for clinical emergency, health management, military safety, biological emergency and other applications.
Core technologies
- Rapid nucleic acid lysis at room temperature within 1 minute
- Ultra fast amplification system in 8-45 minutes
- Integrated automatic data analysis software
- Results immediately printed by built-in printer
Performance
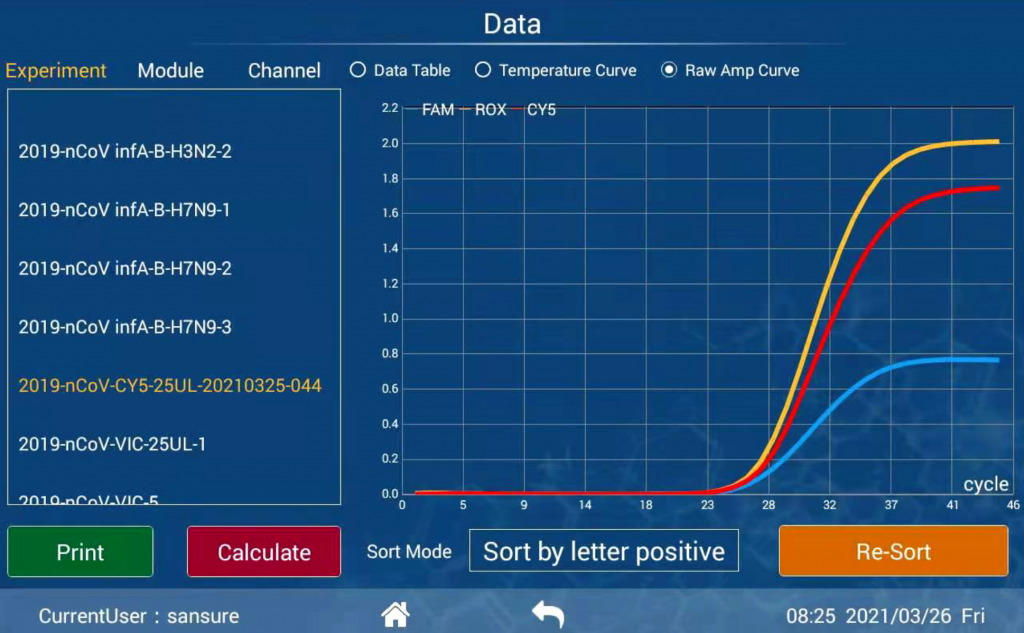 |
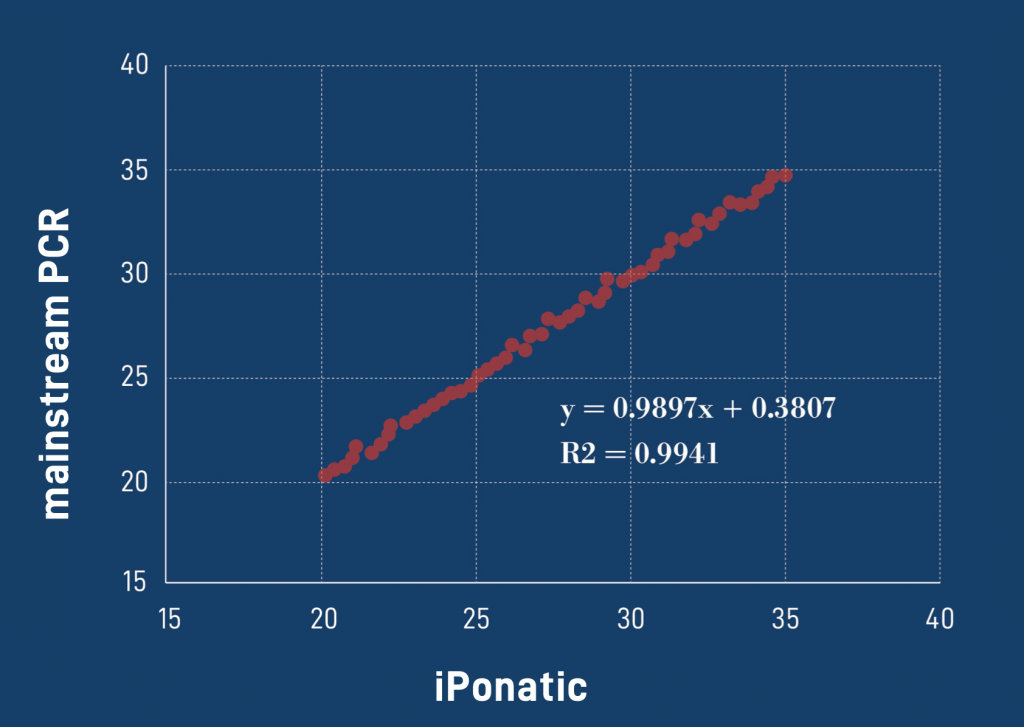 |
Diagnostic results consistent with international mainstream PCR instruments
Parameters
| Model | S-Q31A | S-Q31B | ||
| Detection platform | Real-time PCR | |||
| Detection module | 1 amplification module | 4 amplification modules | ||
| Temperature control | Liquid metal coated ceramic heating / Air cooling technology | |||
| Heating rate | ≥6.0℃ /s (from 50℃ to 95℃ ) | |||
| Cooling rate | ≥2.0℃ /s (from 95℃ to 50℃ ) | |||
| Excitation light source | LED | |||
| Detector | High sensitivity photodiode | |||
| Applicable dyes | FAM | VIC | ROX | CY5 |
| Sensitivity | Can detect single copy gene | |||
| Electrical specification | AC 100-200V 50/60Hz | |||
| Dimension | 230×284×376 mm (L×W×H) | 336.5 × 280 × 435 mm (L×W×H) | ||
| Weight | 9.7Kg | 13Kg | ||
| Qualification | CE | |||
Test menu
Respiratory Infections : SARS-CoV-2, SARS-CoV-2/FluA/B, MP, ADV, BP….
HPV Infections : HrHPV, HPV 13+2, HPV 16/18, HPV 6/11….
Children’s health : EBV, HCMV….
STIs : CT/UU/NG, CT, UU, NG, HSV-2….
Other test projects are under development


