Test for COVID-19 on an exhale? Faster and easier!
It has been nearly three years since the outbreak of COVID-19 in 2020, during which time there have been several epidemic peaks triggered by mutated strains, especially the recent Omicron strain. Clinical data show that the Omicron strain is far more potent than influenza and other mutant strains of COVID-19, and that the strain can spread to about 9.5 people without proper protection, so our prevention and control efforts must race against the virus to identify the initial source of infection early.
China has adopted the dynamic zero-COVID measures and has also achieved good results. The core concept of “dynamic zero” is to take proactive measures to quickly detect and extinguish an emerging epidemic before it grows, to prevent the spread of epidemic diseases in community clustered network. Delayed treatment in outbreaks due to waiting for molecular testing results has been frequently reported recently, and there are also other incidents reported that show the importance of the rapid test results of COVID-19 for carrying out outbreak epidemiological investigation, providing clinical treatment and safeguarding people’s life and health.
Rapid detection technology for COVID-19
The current detection techniques for COVID-19 mainly include molecular and antigen tests, and the primary guidelines mentioned in the Chinese guidelines for the diagnosis and treatment recommend molecular diagnostics and antigen screening. The available rapid molecular tests can theoretically produce results in as soon as half an hour, and antigen tests can even produce results in about 15 minutes.
Most tests for COVID-19, whether based on centralized sample delivery to the laboratory or self-administered kits, require collection of samples via nasal or pharyngeal swabs. In that case the standardization of sampling practices has an effect on the test results, and the waiting time for results ranges from ten minutes to hours, sometimes even longer. Is there a faster and easier way to test for COVID-19? Scientists have been working on research and innovation in this area, and the COVID-19 breath test is a very desirable and convenient method.
Test for COVID-19 on an exhale
Pathogen detection on an exhale is based on the principle that rapid replication of viruses and the body’s immune response lead to changes in specific metabolites, and analysis of volatile organic compounds (VOCs) in the human body can detect changes in metabolites, which in combination with mass spectrometry and artificial intelligence analysis enables determining the specific metabolites responsible for the differences between infected and uninfected individuals, thereby detecting viral infections.
This technique is characterized by the following advantages: non-invasive with no need of sampling nasal swabs, pharyngeal swabs, blood or other samples, comfortable and acceptant for patients; fast, producing results in about 3 minutes and allowing cooperation with other testing methods to speed up the testing; sensitive, reported to be more sensitive than the rapid antigen test; safe, making it easier to test by a non-invasive way while reducing the risk of pathogen spread in the environment; convenient, portable with a small size and applicable to more scenarios flexibly.
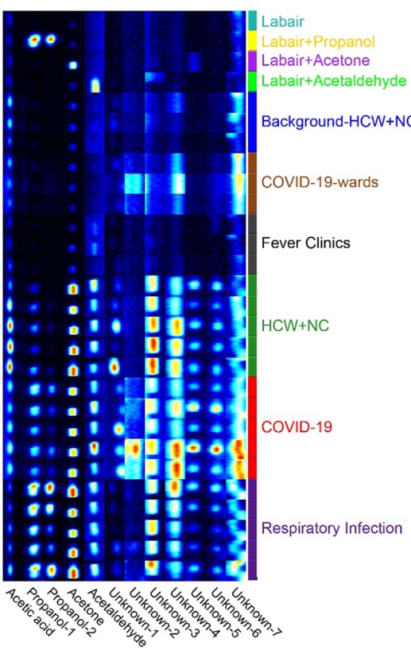
Figure 1: GC-IMS detection of VOC types in different groups
The COVID-19 mass spectrometry of exhaled breath has been reported in previous studies, and most recently COVID-19 screening using breath-borne volatile organic compounds (J Breath Res. 2021 Oct 22;15(4)) was published in the journal J Breath Res. Yao et al. found in their study of COVID-19 patients, patients with respiratory tract infection and that control group that acetone and other volatiles in patients with COVID-19 were significantly lower than those in other populations, and that supervised machine learning models using 12 key endogenous VOCs were able to significantly distinguish those with COVID-19 from the other cohorts.
Test for COVID-19 on an exhale approved in many places
The response to COVID-19 has facilitated the development of industries related to detection and control of viruses, enabling rapid translation of scientific research into practical products, as exemplified by the rapid development and launch of previous mRNA vaccines.
On May 17, 2021, the Health Sciences Authority (HSA) of Singapore granted a provisional authorization for a new exhaled gas detector, “BreFence Go”, for COVID-19 detection, which collects exhaled gas from the subject through a sampler and then feeds it into a proton transfer reaction time-of-flight mass spectrometer (PTR-TOFMS) for detection and screening.
Figure 2: GC-IMS detection of VOC types in different groups
On April 14, the U.S. Food and Drug Administration (FDA) approved an emergency use authorization for a COVID-19 breathalyzer. It was the first exhale type COVID-19 tester based on GC-MS principle approved by the FDA, which is another example of scientific research translation in the field of COVID-19 detection products.

Figure 3: FDA approves emergency use authorization for COVID-19 breathalyzer
The device is a COVID-19 breathalyzer “PNY-1000” from InspectIR, the size of a carry-on suitcase, which contains a gas chromatography mass-spectrometry (GC-MS) system. It can separate and identify chemical mixtures in the subject’s breath sample. The process from sample collection, analysis, testing to reporting results takes about three minutes to measure the presence of the five aldehydes and ketones VOC compounds associated with COVID-19 and thus determine whether the tested subject is infected.
Results from the FDA’s controlled performance study of the InspectIR COVID-19 breathalyzer on 2,409 subjects showed that the InspectIR COVID-19 breathalyzer tested 91.2% accurately on positive samples and 99.3% accurately on negative samples. And the device has also shown a fairly consistent accuracy in clinical studies against variant strains of Omicron. Its sensitivity is above the WHO standard for antigen detection (80%). According to the FDA, the device can be applied in hospitals, clinics and mobile fast screening stations and other scenarios, facilitating COVID-19 screening in the population.

Figure 4: InspectIR COVID-19 breathalyzer
The approval of this new test is another example of the medical community’s commitment to rapid and innovative COVID-19 diagnostics. Supporting the development of COVID-19 detection technologies and advancing technologies that help respond to current epidemics will enable better response to public health emergencies. China has also been increasing its investment in scientific research in recent years, and actively carried out innovative research and development to help advance international medical science and technology.
Note: The picture comes from the Internet. If there is any infringement, please contact the author to delete it.
Disclaimer: All the publications on this website, where the source is indicated, are copyrighted by the original source and do not represent the position of this website.