WHO: Attention should be paid to the detection and triage of COVID-19 and influenza during the epidemic
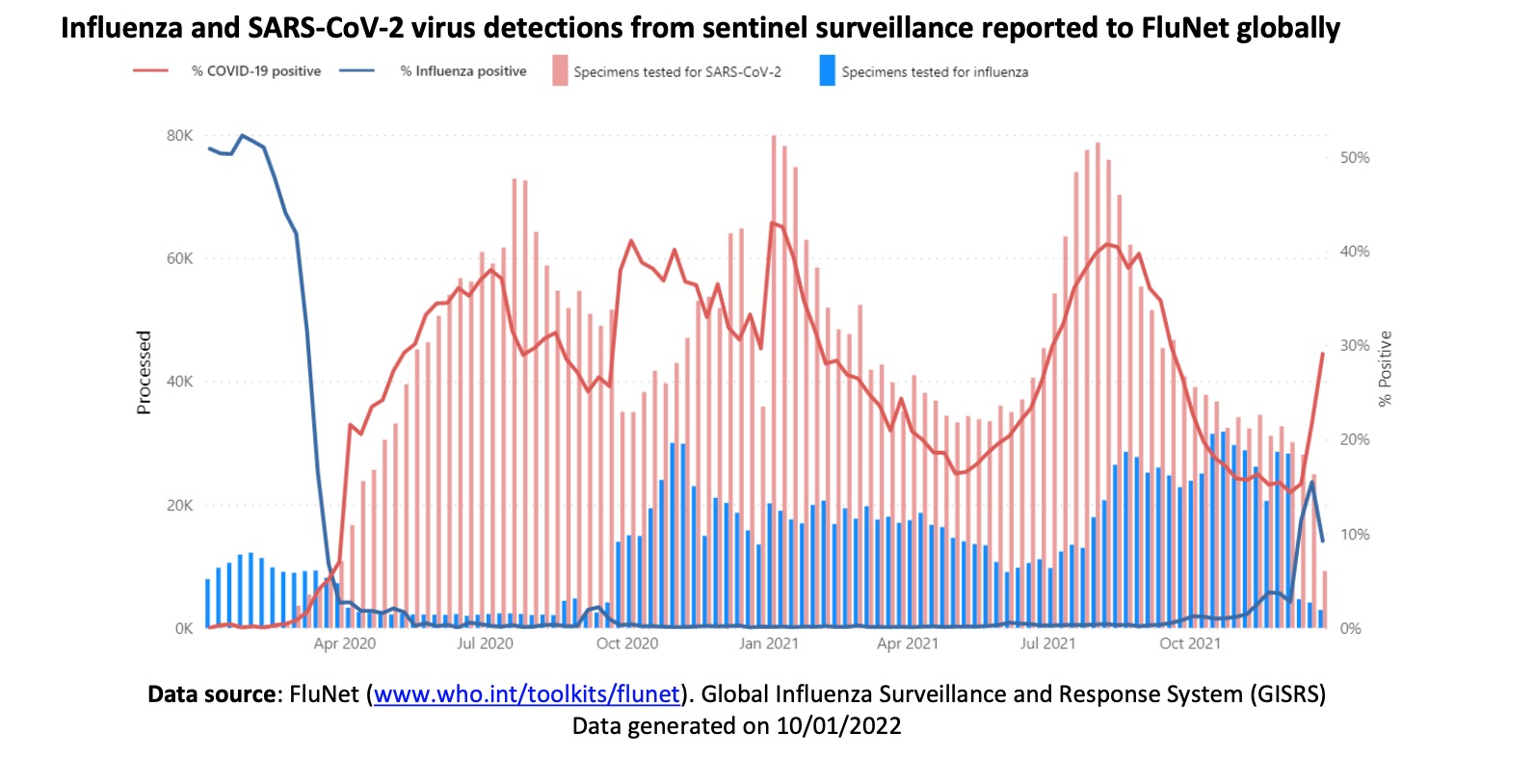
From the beginning of the COVID-19 to the summer of 2021, the global seasonal influenza cases have drastically decreased in comparison with that of previous years thanks to the strict public health and social measures adopted by countries around the world.
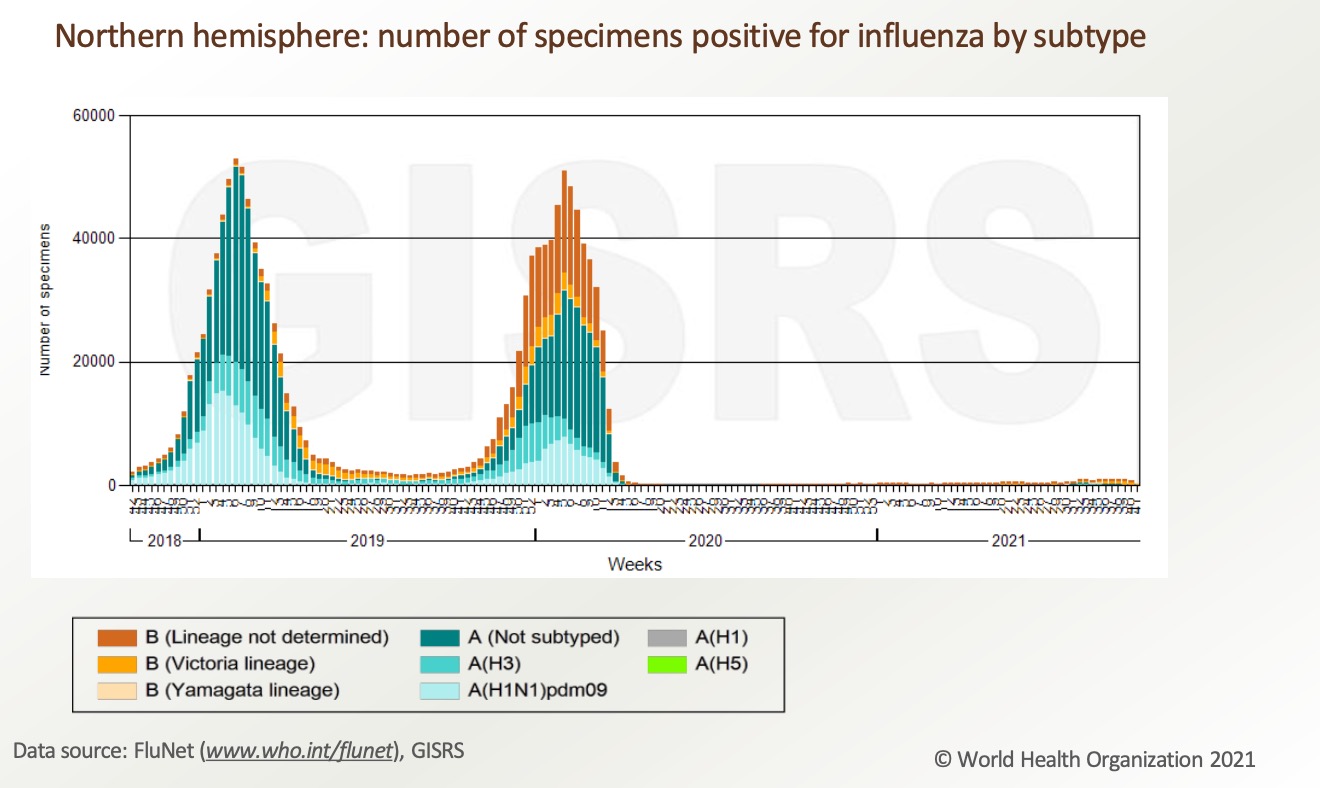
However, influenza cases increase with the relaxation of epidemic prevention in many countries by the end of 2021. Besides, the population’s immunity to influenza may be weakened due to the previous strict quarantine measures reducing people’s exposure to influenza. This may further affect the incidence of seasonal influenza in the future. Based on global influenza surveillance data, the influenza virus infection rate increases in the fall of 2021.
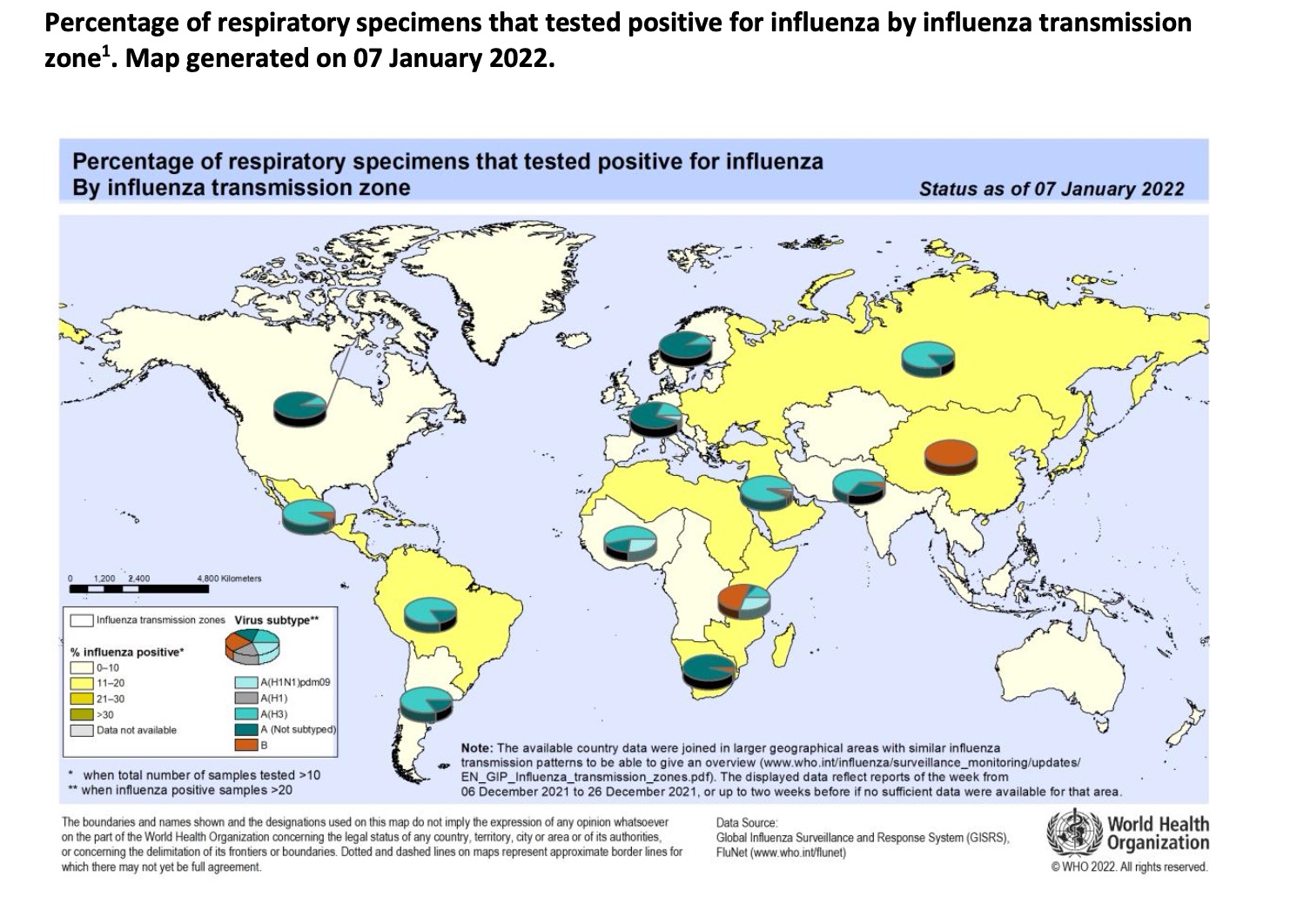
From December 6, 2021 to December 26, 2021, WHO tested more than 522,595 samples from 110 countries or territories, and a total of 27,153 people were positive for influenza virus, of which 19,980 (73.6%) were influenza A and 7,173 people (26.4%) influenza B. Among the influenza A viruses, 352 were influenza A (H1N1) pdm09 and 7625 were influenza A (H3N2). Among the well-characterized influenza B viruses, 3 belonged to B-Yamagata and 6819 belonged to B-Victoria.
The overall influenza activity around the world begins to recover and continues to increase, especially in the temperate regions of the northern hemisphere. In some countries, influenza activity reaches levels seen during the same period the year before COVID-19.
In North America, although overall levels remain low, influenza hospitalizations increase with the increase of influenza A (H3N2)-based testing. In particular, the influenza infection rate in the United States has been similar to that before the COVID-19 epidemic and has exceeded the warning line, while the percentage of deaths caused by pneumonia, influenza or COVID-19 in the United States has above the historical data.
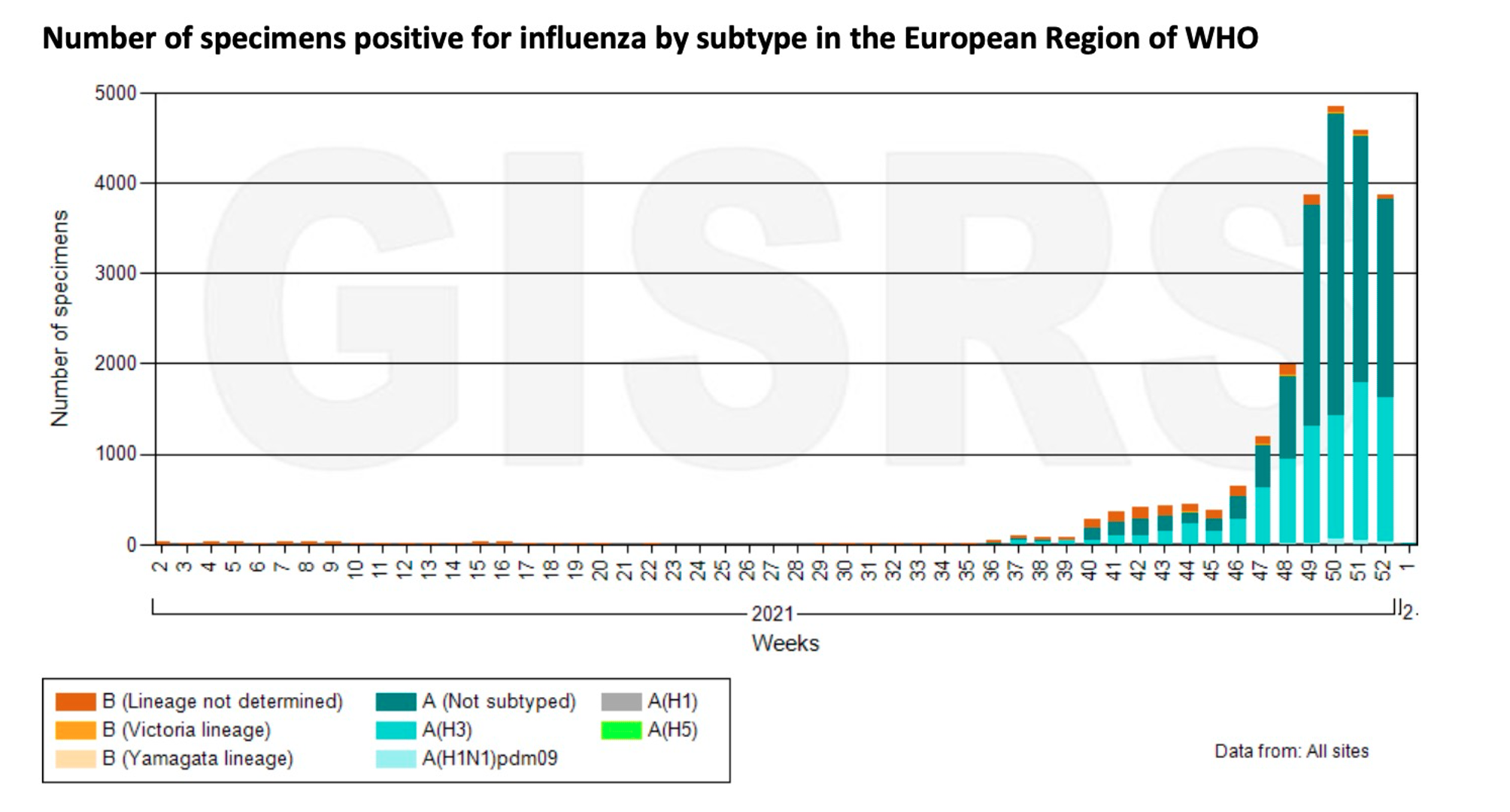
In Europe, influenza activity has increased across the European region, dominated by influenza A (H3N2). Influenza positivity rates at European sentinel sites are above 10% for two consecutive weeks. Therefore, experts say that the European flu season is coming. Influenza activity also exceeds seasonal thresholds in several regions of Russia and France.
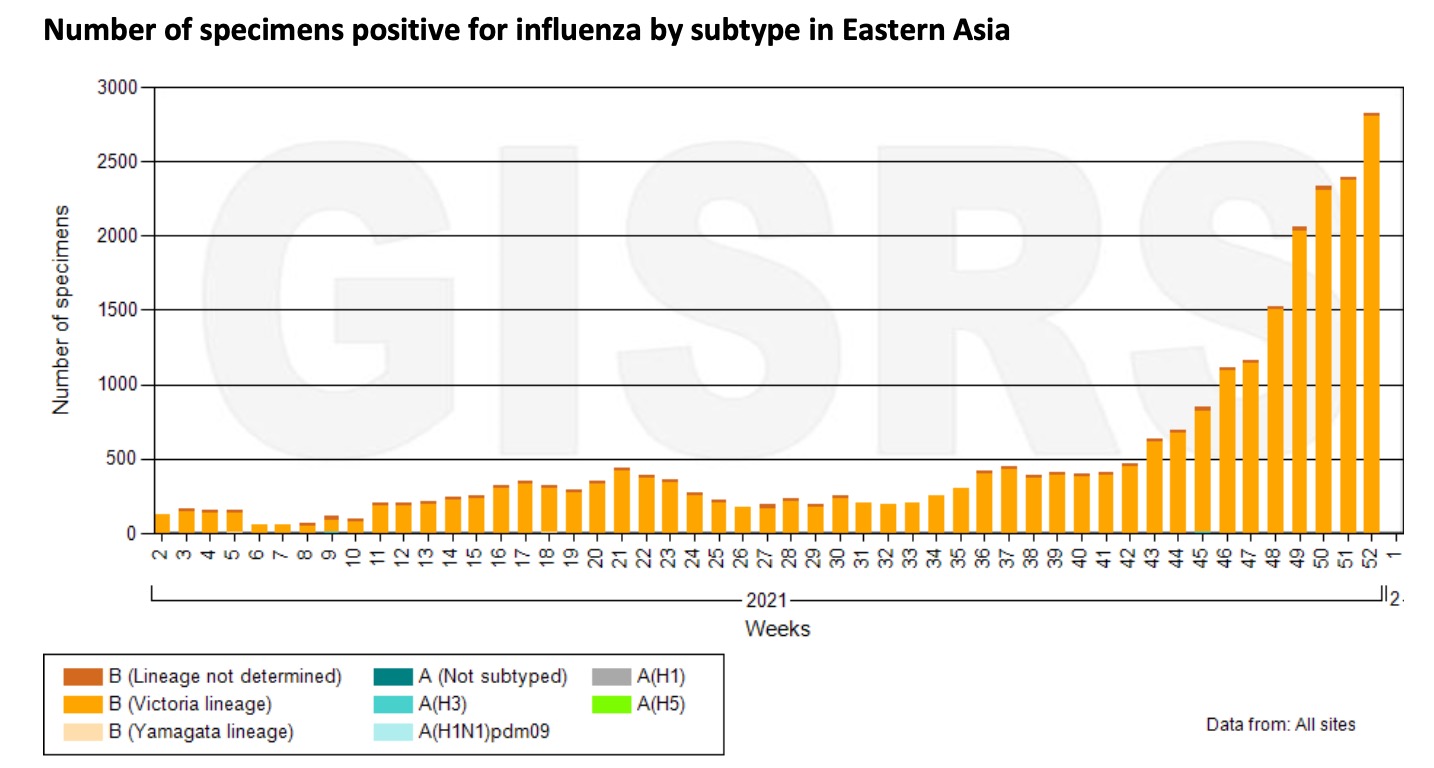
In East Asia, influenza activity continues to trend upward. Among them, the number and positivity rate of influenza B in northern and southern provinces of China continues to increase and reaches the level before the outbreak of the COVID-19. China is currently dominated by the B-Victoria virus.
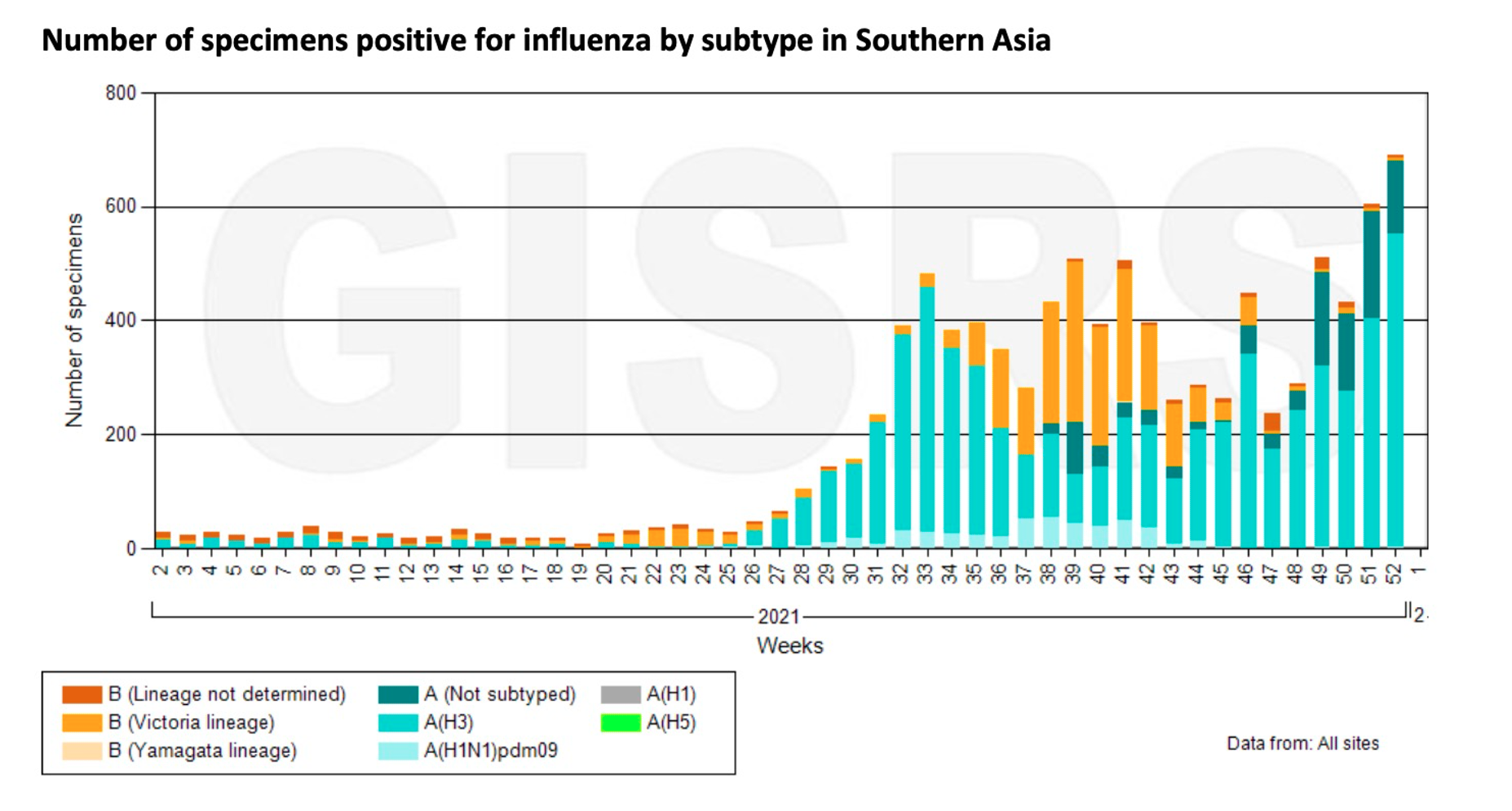
Furthermore, in addition to Africa, regions with increased influenza activity also include South America, South Asia, and Southeast Asia.
There are 1 billion seasonal influenza cases each year worldwide, with 3-5 million severe cases and as many as 650,000 respiratory-related deaths. Influenza viruses can cause acute infections. Viral shedding begins approximately 1 day later in humans and peaks before symptoms appear. Typical flu symptoms include sudden fever, muscle pain, headache, and exhaustion, peaking 2-3 days after infection and resolving within 1-2 weeks. However, in a few cases, influenza virus can cause bacterial infection or worsening of cardiovascular and respiratory diseases, etc., causing influenza complications and even death in severe cases.
Influenza viruses are highly contagious and have caused four pandemics in human history, including the 1918 “Spanish Flu” that killed more than 50 million people worldwide. For most outpatients, the diagnosis is clinical, empirical, without laboratory testing. Laboratory tests are useful for the diagnosis and precise treatment of hospitalized patients with suspected influenza. Many of the marketed influenza rapid molecular diagnostic products are extremely valuable for the treatment of clinical influenza patients. These rapid diagnostic techniques can be performed at the point of care to provide quick and accurate results. Four approved anti-influenza drugs such as oseltamivir, peramivir, baloxavir, and zanamivir may be used for influenza patients within 48 hours of symptom onset. Antiviral therapy works best when used within 24 hours of symptoms appearing. Anti-flu medicines can shorten the duration of illness by about 24 hours and reduce the risk of serious complications.
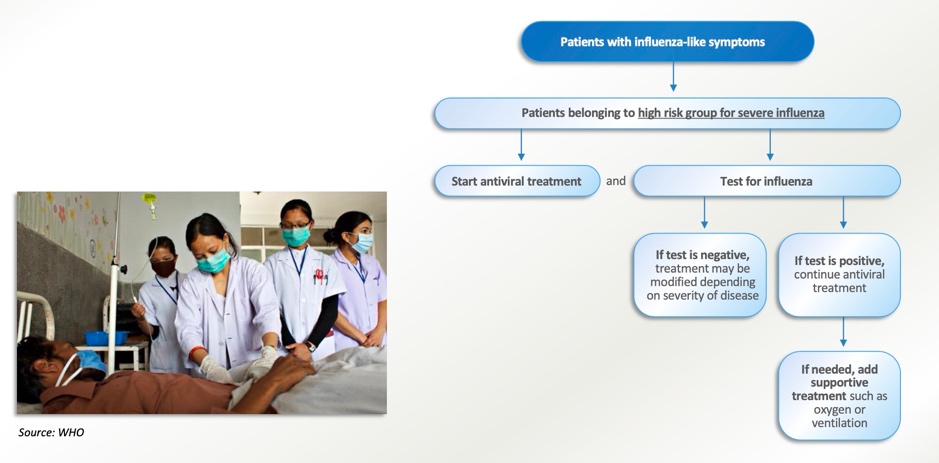
WHO advises countries to prepare for a co-circulation of influenza and SARS-CoV-2 as the positivity rate of influenza testing continues to increase during the COVID-19 pandemic. Countries are encouraged to intensify comprehensive surveillance for both influenza and SARS-CoV-2, and intensify influenza vaccination to prevent severe illness and hospitalization from influenza. Clinicians should consider influenza in the differential diagnosis, especially in high-risk groups for influenza, and provide testing and treatment based on national guidelines.
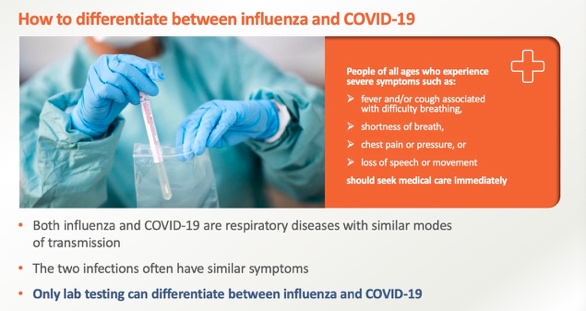
The WHO calls for a triage of influenza and SARS-CoV-2 testing. Influenza and COVID-19 are both respiratory illnesses that spread in similar ways, and the two viral infections often have similar symptoms, and only laboratory tests can tell the difference between influenza and COVID-19. Sansure’s SARS-CoV-2 and Influenza A/B Virus Nucleic Acid Diagnostic Kit can simultaneously detect and identify the SARS-CoV-2, influenza A, and influenza B viruses. The kit is effective for the prevention and control of influenza virus during the epidemic.
Note: The picture comes from the Internet. If there is any infringement, please contact the author to delete it.
Disclaimer: All the publications on this website, where the source is indicated, are copyrighted by the original source and do not represent the position of this website.