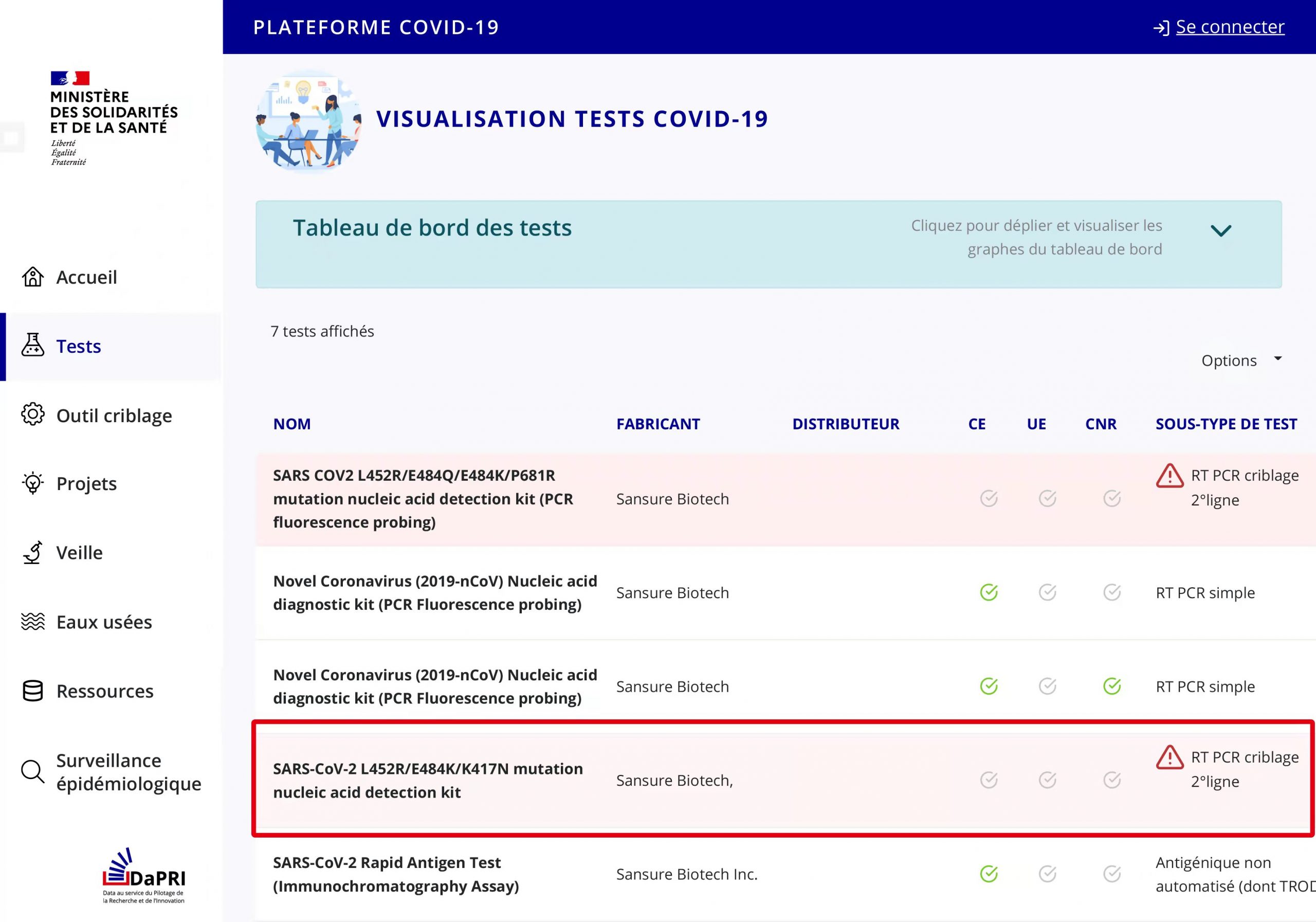
Sansure Biotech’s SARS-CoV-2 variant diagnostic kit received marketing authorization approval from Ministère des Solidarités et de la Santé (Ministry of Solidarity and Health)
Sansure Biotech’s SARS-CoV-2 L452R/E484K/K417N Variant Diagnostic Kit received marketing authorization approval from Ministère des Solidarités et de la Santé (Ministry of Solidarity and Health) the other day. This is Sansure Biotech’s fifth SARS-CoV-2 detection product that has received marketing authorization approval from Ministère des Solidarités et de la Santé.
The Omicron variant of SARS-CoV-2 is now spreading rapidly and rampantly in France. According to the data released by Ministère des Solidarités et de la Santé on the 5th day of this month, the daily confirmed cases of COVID-19 soared above 330,000, hitting a new record high in the country.
In consideration of the rampant spreading of COVID-19 in France and in line with the guidance updated by Ministère des Solidarités et de la Santé on December 17, 2021 for the use of the French National SI-DEP Platform, Sansure Biotech has successfully developed a highly-precise, premium quality, and regulatory compliant diagnostic kit for this variant in merely 10 days by virtue of the company’s fast response, timely tracking, and skillful product development team, and has successfully get emergent marketing authorization approval from Ministère des Solidarités et de la Santé for preliminary diagnosis of the Omicron variant of SARS-CoV-2 as required by the guidance issued by Ministère des Solidarités et de la Santé.

Europe is among the regions being focused by Sansure Biotech in its efforts to support the curbing of the pandemic of COVID-19 worldwide. Sansure Biotech has received favorable comment from the French government, medical institutions and healthcare professionals for its provision of SARS-CoV-2 PCR diagnostic service to tens of millions of people in France since the epidemic of COVID-19 in the country in 2020. Sansure Biotech will continue to provide reliable solutions to support the prevention and control of COVID-19 in France and other European countries.





