Acute severe hepatitis in children during the COVID-19 outbreak: adenovirus infection calling for attention!
According to statistics released by the WHO on April 23, recently, a total of 11 countries (especially the UK) have reported at least 169 cases of acute hepatitis of unknown cause in children. Along with the increase in the number of related cases, it is reported that one child has died of this disease. So far, the cause of this hepatitis still remains unclear. Laboratory testing has excluded the possibility of this disease having been caused by hepatitis virus A, B, C, D, or E. Data released by the UK Health and Safety Executive (HSE) indicate that adenovirus testing has produced positive results in 77% of the cases.
About adenovirus
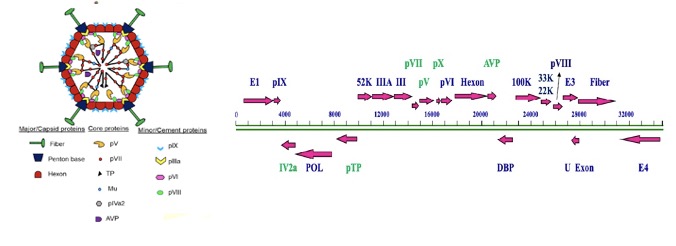
Adenovirus (adv) is a non-enveloped icosahedral virus with double-stranded DNA genomes. Since being isolated from human tonsils and adenoids by Wallace P. Rowe in 1953 and named as thus, adenovirus has had more than 120 specific adenoviral serotypes identified in humans, mammals, birds, fish, and reptiles. They have been classified into seven subgroups, i.e., A, B, C, D, E, F, and G.
Structure of adenoviral genomes
Adenovirus presents a non-enveloped spherical structure. Adenoviral virions often show a crystalline arrangement in infected nuclei. Adenovirus also contains 26–48 kb linear double-stranded DNA genomes. Depending on the time after infection, it produces transcripts of early-stage (E), interim-stage (I), and late-stage (L) regions. Late-stage (L) region encoded structural proteins and non-structural proteins are involved in capsid formation, DNA embedding, and progeny adenovirus maturation. Adenoviral proteins include nucleocapsid proteins, core protein, and secondary proteins. Core proteins include pVII, pV, Mu (pX), pIVa2, terminal protein (TP), and AdV-encoded protease.
Epidemiology of adenovirus
Adenovirus is a seasonally transmitted virus that prevails from February to April. It is a common human-infected virus that is transmitted mainly via respiratory droplets, and also through feces. Adenovirus infection is often seen in military recruits, students, and nursery and kindergarten children, especially children below 4, elderly people, and people with compromised immunity. The clinical manifestations of adenovirus infection are very extensive, ranging from asymptom to life-threatening severe respiratory infections and tissue-invasive infections. Different adenoviral serotypes may give rise to a series of clinical diseases, such as respiratory tract infection, respiratory system diseases, keratoconjunctivitis, gastroenteritis, and urinary infection.
Immunocompromised patients (such as patients who have received stem cell transplantation or organ transplantation) are susceptible to invasive adenoviral infection. In patients with HIV infection or patients who have undergone parenchymatous organ transplantation or allogenic hematopoietic stem cell transplantation, adenovirus may reactivate or cause new infections, resulting in multi-organ infections like enteritis, hemorrhagic cystitis, hepatitis, and pneumonia. The infection rate of adenovirus can reach as high as 3.0%–21.0%, and the mortality can reach 7.7%–38.0%. Invasive adenoviral infection does more harms to children.
An acute severe hepatitis of unknown cause has been detected in children in many countries. Adenovirus testing has produced positive results in 77% of the cases.

The children with the acute severe hepatitis of unknown cause reported in the UK are mostly 2–5 years old, and their main symptoms are vomiting, skin jaundice, and yellowing of eye whites. The level of alanine transaminase (ALT) in blood has exceeded 2,000 IU/L (normal range: 10–40 IU/L) in most children patients. Previous reports suggest that, when immunocompromised young people have invasive adenoviral infection in the liver, the level of ALT in their blood also gradually increases and exceeds 2,000 IU/L.
To ascertain the cause of the acute severe hepatitis, investigators inquired and investigated the guardians of the children patients. After excluding the possibility of the disease having been caused by toxic substances in food, water, or toys, they further precluded any association with COVID-19 vaccines, as none of the patients had been vaccinated. When the scope of testing was widened, it was found that adenovirus testing produced positive results in five of 13 Scottish children with the acute severe hepatitis. US Ministry of Health officials later confirmed that five of nine US children patients were found to be adenovirus type 41 (Ad41) positive.
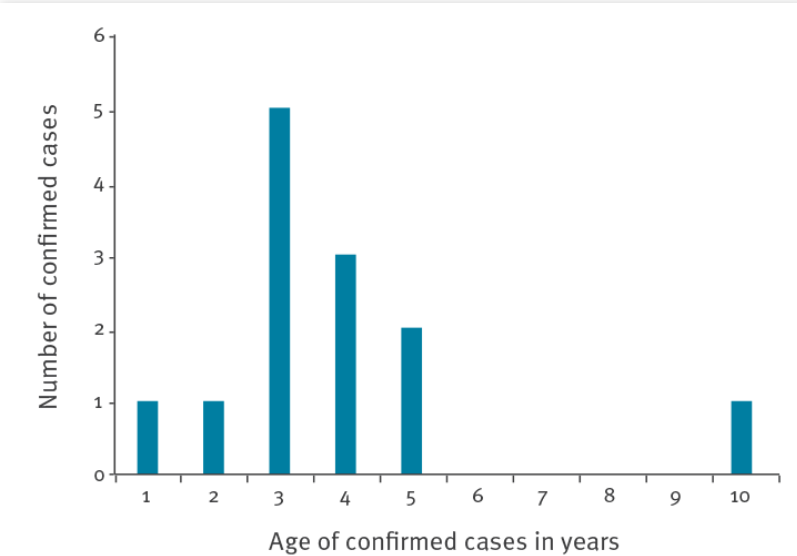
Age distribution of 13 children diagnosed with unexplained severe hepatitis in Scotland, UK
Adenovirus testing
So far, there is no adenovirus-specific vaccine, or approved specific medicine for treatment of adenovirus infection. For immunocompromised people, especially children, early diagnosis and timely supportive treatment are particularly important. Some large children’s hospitals even recommend adenovirus testing as a screening item for children with respiratory tract infection. Adenovirus testing can be performed by isolated cell culture, serological identification, and PCR. However, due to the long time of adenovirus cell culture, the limited value of antibody test in immunosuppressed people, and the lack of quantifying and genotyping functions, the actual clinical application effects are undesirable. By contrast, RT–PCR can be directly used to test nucleotide sequences specific to adenovirus pathogens, with high specificity and sensitivity and desirable genotyping advantages. This also explains why RT–PCR is highly favored. The European Society for Blood and Marrow Transplantation (EBMT) recommends that QPCR be adopted to diagnose and monitor the ADV in the blood and feces samples of child and adults receiving transplantation, so that interventions can be provided as early as possible.
For the purpose of adenovirus testing, Sansure Biotech has launched two testing products, including the Six Respiratory Pathogens Nucleic Acid Diagnostic Kit launched in 2021 and the Respiratory Adenovirus DNA Diagnostic Kit launched this year. The two adenovirus testing kits have covered the common adenovirus subtypes (lowest limit of detection: 200 copies/ml), and are compatible with common PCR instruments on the market. Relying on rapid screening and timely control, we can detect such infections in the early stage, thus contributing to the building of a precision diagnosis and treatment system.
Note: The picture comes from the Internet. If there is any infringement, please contact the author to delete it.
Disclaimer: All the publications on this website, where the source is indicated, are copyrighted by the original source and do not represent the position of this website.